S.-M. Omrani,1,2 H. Vatandoost,2 M.A. Oshaghi 2 and A. Rahimi 3
استجابات الأنوفيلة الاصطفانية بالانجذاب نحو ثنائي أكسيد الكربون وحمض الـ - L لاكتيك: دراسة بمقياس الشم
سيد محمد عمراني، حسن وطن دوست، محمد علي عشاقي، عباس رحيمي
الخلاصـة: إن إفراغ ثنائي أكسيد الكربون وحمض اللاكتيك في الزفير وفي العرق يعطي شارات شَمِّية للبعوض تمكِّنه من العثور على الناس ولَدْغهم، ولو أن أنواع البعوض يختلف بعضها عن بعض في هذا الصدد. وتستقصي هذه الدراسة الاستجابات الانجذابية بعكس الريح للأنوفيلة الاصطفانية، في شكلها الأليف للقاذورات، وهو من النواقل الهامة للملاريا في آسيا، نحو ثنائي أكسيد الكربون وحمض الـ - L- لاكتيك، ضمن شروط المختبرات. ففي حين أدت جرعة دنيا من ثنائي أكسيد الكربون (90 جزءاً بالمليون) إلى تنشيط البعوض، فإن جرعة تعادل عشرة أضعاف ذلك أدت إلى تثبيطه. ولم يؤد حمض الـ - L- لاكتيك بحدِّ ذاته إلى أي تأثير ذي شأن، إلا أن إضافة 6 ميكروغرام/دقيقة من حمض الـ - L- لاكتيك إلى مقدار من ثنائي أكسيد الكربون يتراوح بين 90 و410 جزءاً بالمليون أدى إلى اجتذاب البعوض. وتقدم هذه النتائج المزيد من الدعم للنظرية التي تقول بأن لثنائي أكسيد الكربون دوراً هاماً في سلوك البعوض للبحث عن البشر، وتقترح أنه ربّما يكون لحمض الـ - L- لاكتيك دور أكثر شأناً من دور ثنائي أكسيد الكربون في اجتذاب الأنوفيلة الاصطفانية.
ABSTRACT Excretion of carbon dioxide and L-lactic acid through exhalation and perspiration provides olfactory signals to mosquitoes which allow them to find and bite humans; however, mosquito species differ in this regard. This study investigated upwind responses of Anopheles stephensi, mysorensis form, an important malaria vector in Asia, to carbon dioxide and L-lactic acid under laboratory conditions. While a minimal dose of carbon dioxide (90 ppm) activated the mosquitoes, 10 times this amount suppressed them. L-lactic acid alone did not produce a significant effect by itself, but addition of 6 µg/min of L-lactic acid to a range of 90 to 410 ppm carbon dioxide resulted in attraction. The results provide further support for the hypothesis that CO2 plays an important role in the host-seeking behaviour of zoophilic mosquitoes, and suggests that L-lactic acid might play a more critical role than CO2 in the attraction of An. stephensi.
Réponses sous le vent d'Anopheles stephensi au dioxyde de carbone et à l'acide lactique L une étude en olfactomètre
RÉSUMÉ L'excrétion de dioxyde de carbone et d'acide lactique L par expiration et par perspiration génère des signaux olfactifs qui permettent aux moustiques de repérer et de piquer les humains ; toutefois, toutes les espèces de moustiques ne réagissent pas de manière identique. La présente étude a analysé les réponses sous le vent d'Anopheles stephensi, de type mysorensis, un important vecteur du paludisme en Asie, au dioxyde de carbone et à l'acide lactique L en laboratoire. Alors qu'une dose minimale de dioxyde de carbone (90 ppm) rendait les moustiques actifs, la même dose multipliée par dix avait l'effet inverse. L'acide lactique L seul ne produisait pas d'effet significatif en soi, mais l'association de 6 µg/min d'acide lactique L à une quantité de 90 à 410 ppm de dioxyde de carbone attirait les moustiques. Ces résultats renforcent l'hypothèse selon laquelle le CO2 joue un rôle important dans le comportement de recherche d'hôte chez les moustiques zoophiles, et suggèrent que l'acide lactique L pourrait jouer un rôle plus important que le CO2 dans l'attirance d'Anopheles stephensi.
1Department of Medical Parasitology, School of Medicine, Shahrekord University of Medical Sciences, Shahrekord, Islamic Republic of Iran.
2Department of Medical Entomology and Vector Control; 3Department of Epidemiology and Biostatistics, School of Public Health & National Institute for Health Research, Tehran University of Medical Sciences, Tehran, Islamic Republic of Iran (Correspondence to H. Vatandoost:
Received: 29/06/10; accepted: 28/11/10
EMHJ, 2012, 18(11):1134-1142
Introduction
Malaria remains a major public health problem in southern part of the Islamic Republic of Iran; about 80% of all malaria cases in the country are reported from this region and there are 6 anopheline mosquitoes known to be malaria vectors [1–5]. Anopheles stephensi is an important malaria vector throughout south Asia, including the Indo–Pakistan subcontinent and the Middle East. However, it is one of the least anthropophilic malaria mosquitoes in the world [6]. It has 3 biological forms, type, intermediate and mysorensis.
Yearly, a large number of healthy years of human life are lost due to mosquito-borne diseases, including malaria. Excretion of waste materials through exhalation and skin emanations of sweat and respiration accompanied by the act of normal floral microorganisms unintentionally provide potent olfactory signals, inviting physiologically-competent mosquitoes to find and bite humans. Several studies have shown that mosquitoes exploit carbon dioxide (CO2) as a chemical cue in their long-range orientation towards a potential host [7–10].
At the same time, while L-lactic acid alone is reported to be only slightly attractive, neutral or even repellent to mosquitoes, it has been shown that in combination with CO2 it attracts them. This synergistic effect was first noticed for Aedes aegypti and then for An. gambiae [11], and Kline et al. showed that this binary blend increases catches of certain dipterans, including mosquitoes [12]. However, Stryker and Young did not detect this synergistic effect in the field except for Ae. vexans [13].
Although, these comparative studies shed light on principles governing the host-seeking behaviour of mosquitoes, it is clear that practical application of this knowledge in surveillance programmes or effective control measures needs further specific information of a given mosquito species in its locality.
There are 3 biological forms of An. stephensi; the mysorensis form is colonized and considered the main malaria vector in the country. Therefore, the aim of this study was to investigate the upwind responses of the mysorensis form of An. stephensi to CO2 and L-lactic acid within a dual-choice olfactometer.
Methods
Mosquitoes
The An. stephensi used was the mysorensis form. It originated from Iranshahr, Islamic Republic of Iran and has been kept in the insectary of Tehran University of Medical Sciences, School of Public Health, since 2006. For this study a specific colony of this mosquito was used that was established under 29 ± 1 °C, 80% ± 5% relative humidity, light/dark cycle 12:12 h conditions, with a simulated nightfall at midday. Two small stock cultures of adult females were offered blood from Guinea pigs for 45 minutes biweekly in an alternative schedule. Eggs were laid on wet filter paper, hatched in water bowls and transferred to water-filled plastic trays the next day. Larvae (with density of 1 per mL of dechlorized tap water) were fed with Tetramin® fish food based on a fixed local protocol. Pupae were collected daily from the trays and transferred in populations of 1000–1500 into 30×30×30 cm gauze-covered adult cages. Adults were kept with access only to 10% glucose solution. All experiments were done on 4–5-day-old 8–10-h sugar-deprived host responsive female mosquitoes exactly during the first hour of the middle third of the scotophase. These mosquitoes were put in a population of 10 in 5 small cages and transferred to the laboratory in an opaque plastic box matted with wet tissues.
Olfactometer, bioassays and procedures
A slightly modified Geier type dual-port olfactometer made by the authors was used [14]. Details of the apparatus have been described elsewhere [15]. In brief, charcoal-filtered, humidified (50% ± 2%) and warm air (29 ± 0.1 °C) was led via PVC pipelines to the olfactometer arms (15×25 cm acrylic cylinders, 15 cm apart). Wind speed in the cylindrical wind tunnel was kept constant at 0.4 m/s. Light from 2 25-watt incandescent bulbs hanging 80 cm above the olfactometer provided 11 lux scattered dim light during the experiments. Two 50×150 cm white plastic sheets at the bilateral sides of the wind tunnel prevented undesirable optical stimulation of mosquitoes.
A precise amount of the chemical stimuli regulated by fine flow meters and in a non-oscillating gaseous form were conducted to the treatment arm through silicone pipelines (5 mm internal diameter, 100 cm length). The flow meter for flow rates above 1000 mL/min was from a different manufacturer (MBLD Instrument Company, China). These chemicals were injected individually or in combination using separate large single bore steel needles piercing a circular rubber septum over a small hole located 3 cm from the treatment arm aperture. According Geier et al. this type of injection generates a homogeneous plume [14]. All injections were performed just a few seconds prior to releasing mosquitoes into the olfactometer.
Any stimulus dosage was tested in 2 consecutive experiment sets, each comprised 1 trial of no chemical stimulus injection as a control followed by 4 trials of test material. Injections were alternated between right and left arms to avoid a systematic bias. In each trial a small cage containing 10 fresh mosquitoes was connected to the downwind end of the wind tunnel. After 1–3 minutes acclimatization, mosquitoes were allowed to freely choose olfactometer arms during 1 minute experimentation time after to injection of test material. Mosquitoes were removed with an electrical vacuum cleaner at the end of each experiment. The experimenter wore cotton gloves throughout the experiments and avoided touching inner parts of the olfactometer.
Odours
CO2 and L-lactic acid were tested individually and in combination within the olfactometer. Different concentrations of carbon dioxide were produced by serial injections of 50, 150, 300, 600, 900, 1200 and 2400 mL/min 2% carbon dioxide from a pressurized gas cylinder (Anagaz Co., Tehran) into the treatment arm. An infrared hand-held CO2 analyser (Testo 535, Germany) was used to identify concentrations of these flows in the wind tunnel.
Various concentrations of L-lactic acid were used derived from passing incremental flows of clean dry air (from the air supply of the olfactometer) at 50, 150 and 450 mL/min through 100 mL of logarithmic dilutions of 1:10 or 1:100 aqueous L-lactic acid solutions (original concentration from Merck, Germany) in a 250 mL gas washing bottle. A rough estimation of the exact amount of L-lactic acid released in these flow rates was possible after Geier et al.[14]; therefore measurement attempts were made only for the most effective dosage due to difficulty in lactic acid detection at very low concentrations. To do this, the output of a certain flow of bubbling air in 200 mL of diluted L-lactic acid was passed through 2 serial gas washing bottles containing 100 mL distilled water over a 50-minute period. Trapped L-lactic acid in these 2 bottles was titrated by 0.001 N sodium hydroxide and 0.001 N hydrochloric acid. An estimate of the total amount of L-lactic acid was made from extrapolation of the rate of decrease of dissolved L-lactic acid in these bottles.
Statistical analysis
The proportion of mosquitoes that left the small release cage and that were trapped inside either arm of the olfactometer at the end of 1 minute experimentation time represented activation (%) and attraction (%) to the treatment or control arms respectively. Data for each trial were entered in SPSS, version 11.50. Comparison of a series of variables was done by nonparametric Kruskal–Wallis test (α = 0.05), as needed.
Results
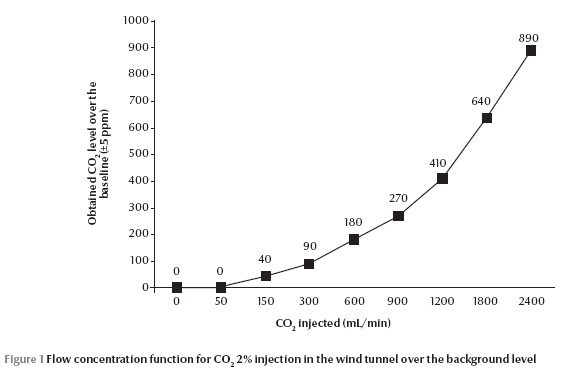
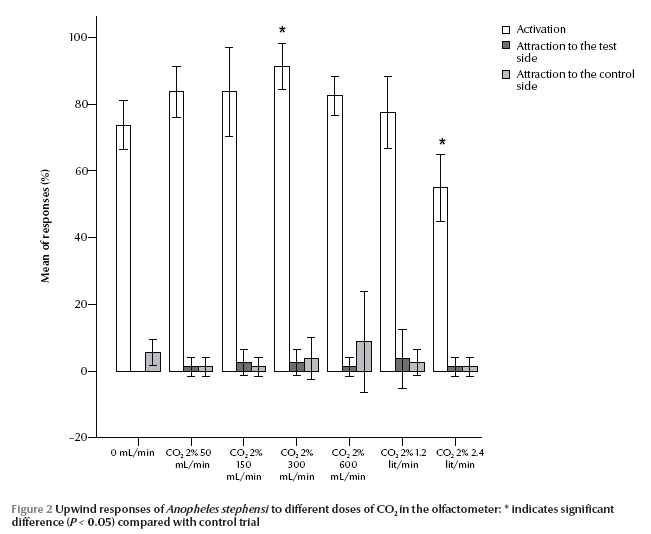
The infrared CO2 analyser did not detect any rise in carbon dioxide (0 ppm) at 50 mL/min 2% CO2 injection over the ambient level (400 ppm) (Figure 1). The most consistent part of this flow concentration function was for 150 up to 900 mL/min, equal to 40 to 270 ppm respectively; the greater the flow of injected CO2, the greater the deviation from the expected concentration value. The highest and the lowest activation of An. stephensi was observed at 300 mL/min (90 ppm) and 2400 mL/min (890 ppm) 2% CO2 injection respectively (P = 0.003 and 0.005) (Figure 2). However, attraction responses of mosquitoes to CO2 were not significantly different at any concentrations examined or any paired treatment and control arms. In the case of L-lactic acid alone, no stimulus dosage produced a statistically different activation or attraction response (Figure 3). This figure also did not change with proportion of mosquitoes activated in response to any binary blend of CO2 and L-lactic acid (Figure 4). Addition of either 50 or 2400 mL/min 2% CO2 to L-lactic acid at any dilution or flow rate also did not attract mosquitoes at all or the attraction to the treatment arm was not significantly different from the control arm. This corresponds to data from the CO2 analyser and CO2 experiments since the concentration of CO2 in the wind tunnel was no different from the ambient level at 50 mL/min 2% CO2 injection, and injection of 2400 mL/min 2% CO2 (890 ppm) somehow deterred mosquitoes from following the CO2 trail too. On the other hand, injection of either 300 (90 ppm) or 1200 (410 ppm) mL/min 2% CO2 in the treatment arm evoked attraction at almost all injected L-lactic acid dilutions and flow rates. However, the attraction was significantly different from the control are only at the maximum dose of L-lactic acid injection, i.e. 1:10 dilution and 450 mL/min. At this flow rate about 6 µg/min L-lactic acid is released into the olfactometer which is measured by a titration technique.
The mean activation responses of mosquitoes in no-stimulus tests were statistically different from CO2, L-lactic acid, and CO2 plus L-lactic acid experiments (Kruskal–Wallis, P < 0.001) (Table. 1).
Discussion
This study, conducted on the mysorensis form of An. stephensi as a model, provides further support for the common finding that CO2 activates and L-lactic acid in the presence of carbon dioxide attract mosquitoes.
Carbon dioxide experiments
In these experiments mosquitoes were activated with a homogeneous plume of about 0.01% CO2 over the ambient level. To our knowledge this is the first report of activation response of An. stephensi to such a low level of CO2. We also observed that this reaction was relatively diminished with 10 times more CO2, i.e. about 0.1%. Takken et al. used pulses of 5% CO2 instead and found that while individuals of this mosquito species were activated at this human equivalent concentration, An. gambiae ss does not respond to it well [16]. In a similar study, where An. quadriannulatus showed a strong response to CO2 even in the presence of its preferred cow host, An. arabiensis had a poor response [17]. These results are consistent with findings from field studies in which An. gambiae ss and An. arabiensis showed a low level of attraction to CO2 [18]. Even a 5-fold increase in the emission rate of CO2 did not improve attractions of An. gambiae ss and An. arabiensis. There is also evidence that anthropophilic Culex quinquefasciatus responds poorly to CO2 both under laboratory conditions [19] and in the field [20]. In all of these studies, the authors postulated that specialist mosquitoes such as anthropophilic Cx. quinquefasciatus, An. gambiae ss and to some extent An. arabiensis rely on more specific cues like skin emanations to find their preferred human host. But in opportunistic or more generalist species like zoophilic An. quadriannulatus and An. stephensi CO2 could be enough to find a potential host. We believe that our results are in line with these findings and support the hypothesis that the role of CO2 increases with degree of zoophily. Nevertheless, a few issues need to be considered here.
First, although Takken et al. used 4.5% CO2 in their experiments, this concentration decreased upon its injection into the wind tunnel air flow [16]. On the other hand, it is known that the CO2 exhaled by a human is immediately diluted in the ambient air to an estimated concentration of between 0.01% and 1.0% [21]. Therefore, it is likely that the results of these 2 studies are not far different.
Second, it is generally believed that the change in the concentration of CO2 is more important than its actual level and elicits behavioural responses in mosquitoes [22,23]. On the other hand, electrophysiological recording from CO2 sensitive sensilla on maxillary palps of mosquitoes shows that the housing neuroreceptor cells are rapidly adapted to CO2 exposure in a phasic tonic manner. Moreover, the importance of the structure of the odour plume in the upwind responses of mosquitoes has been also illustrated well [14]. Adding to these facts, we frequently observed that some mosquitoes took off with a few seconds delay after confronting the oncoming wave of injected carbon dioxide. All these pieces of evidence together suggest that perhaps the geometry of our wind tunnel, accompanied by the very low concentration of CO2 we used, was such that the generated odour plume was not completely homogeneous. Besides, the odour plume shape and its variability are more important in the attraction of mosquitoes than in their activation.
Third, Grant and O’Connell with the aid of electrophysiological techniques demonstrated that the CO2 concentration–response curves for CO2 receptor neurons in the sensilla basiconica of mosquitoes from 3 different genera, including anthropophilic and zoophilic species, are more or less similar [24]. This implies that behavioural dissimilarity of various mosquitoes, including the mysorensis form of An. stephensi, in responding to a given concentration of CO2 is modulated at the central level.
Fourth, even though Kellogg showed that electrophysiological responses of the phasic peaks of CO2-sensitive receptors in the maxillary palp of Ae. aegypti saturate at levels between 0.0% and 0.5%, the negative effect of CO2 on the activation of An. stephensi at about 0.1% cannot be easily explained [25]. Nonetheless, the structure of the generated odour plume in the olfactometer and the specific sensitivity of the mosquito species examined probably play a part here.
L-lactic acid and L-lactic acid plus carbon dioxide experiments
Based on the work of Geier et al., it might be roughly estimated that we tested 0.018 to 19 µg/min of L-lactic acid in our series of experiments [14]. However, the largest dosage used (450 mL/min of 1:10 L-lactic acid) gave only 6 µg/min of L-lactic acid in our olfactometer as measured by a chemical titration technique. Perhaps the most direct cause of this difference comes from the fact that we applied bubbling air passed in an L-lactic acid solution instead of using the headspace air over it. In any case, part of the examined range overlaps with the rate of L-lactic acid output from human hands and arms, which has been measured to be between 0.38 and 2.2 µg/min [26].
Dealing with the activation responses, it is clear that the means in no stimulus trials were very high (Table 1). It is worth saying here that this was observed despite the experimenter using cotton gloves throughout the experiments, careful avoidance of touching the inner parts of the wind tunnel, occasional washing of the wind tunnel with absolute ethanol, supply of air to the olfactometer from outside the building and finally strict control of wind speed, temperature and humidity in the wind tunnel. Therefore, where mosquitoes are already maximally active, one cannot make inferences on the activating effect of the test stimuli.
L-lactic acid alone in the doses we tested did not attract the mysorensis form of An. stephensi. A few works have previously reported the same result for other mosquito species. Acree et al. showed that attraction of Ae. aegypti to 10 µg of L-lactic acid is not more than 1% in 3 minutes experimentation time [27]. Also, an air stream containing either 153 µg [11] or 1000 µg [28] of L-lactic acid was not attractive for An. gambiae. In the field, L-lactic acid was not able to improve the trapping of mosquitoes of different genera in a CDC light trap either [12]. Catches of Ae. albopictus in traps baited with L-lactic acid were not statistically different from controls in another field study [29]. However, conflicting with these results are a few reports on slight attraction or even repellence to this compound. Geier et al. found that Ae. aegypti was attracted to 3 µg/min of L-lactic acid in the wind tunnel [14]; Williams et al. showed that 4 different geographical strains of Ae. aegypti were attracted to L-lactic acid but that the threshold dose for the same level of attraction was remarkably unequal and ranged from 0.03 to 10.27 µg/min [30]. From these studies, including our experiments, it can be inferred that L-lactic acid alone weakly attracts mosquitoes, if at all, especially anopheline species. Perhaps the main sources of variation in the results are the dosages used, the olfactometer type, the experimentation time and protocol, the structure of the odour plume and the mosquito species and geographical strain. Even the atmospheric pressure on the day of experiment is considered to modulate to some extent the attraction responses of mosquitoes [31].
In our study, only the highest dose of L-lactic acid used, i.e. 6 µg/min in combination with either 90 or 410 ppm CO2, attracted An. stephensi. Inability of the lower doses of L-lactic acid to synergize with CO2 indicates its dose dependency. This synergistic effect has been previously reported for other mosquitoes; 10 µg of L-lactic acid in the presence of 1000 ppm CO2 attracted 29% to 75% of Ae. aegypti in 3 minutes [27]. Nearly the same response has been reported by others [14]. They showed that 8 µg of L-lactic acid in an air current containing 1000 ppm CO2 attracted 86% of Ae. aegypti, considerably higher than the 20% and 41% for either L-lactic acid or CO2 alone respectively.
The synergistic action was observed with a fixed dose of L-lactic acid in contrast to a variable dose of CO2. Such a modulatory effect of L-lactic acid over CO2 may be because the L-lactic acid plays a more critical role than CO2 in the attraction responses of mosquitoes. This hypothesis is supported by the findings that addition or removal of a certain dose of L-lactic acid to or from human skin extracts significantly increases or decreases attraction of Ae. aegypti [32].
Regarding the synergistic effect of L-lactic acid with CO2 2 points should be noted. First, the level of augmented attraction observed under laboratory conditions has never been seen in field studies [12,13,33]. This may stem partly from the strong modifying influence of environmental factors, such as the structure and shape of the odour plume on source-searching behaviour of mosquitoes under uncontrolled conditions. Second, CO2 alone or in combination with L-lactic acid elicited no change in spike frequency of L-lactic acid-sensitive neurons in the antennae of Ae. aegypti, indicating that the behavioural synergism of CO2 and L-lactic acid occurs centrally and not at the primary receptor level [34].
Conclusion
This study provides further support for the hypothesis that CO2 plays a more important role in the host-seeking behaviour of zoophilic mosquitoes than the anthropophilic mosquito species. It also suggests that L-lactic acid might play a more critical role than CO2 in the attraction of An. stephensi as a certain dose of L-lactic acid modulates the effect of a range of doses of CO2.
If field trials verify these findings, this information can be used for the development of species-specific odour-baited entry traps which could provide a better estimation of population dynamics of this malaria mosquito in surveillance programmes at least.
Acknowledgements
The authors are grateful to Dr Patrick Guerin, Department of Animal Sensory Physiology, University of Neuchâtel, Switzerland as his critical discussions and comments on olfaction-based behaviours of mosquitoes. Thanks are also due to Dr Thomas Kröber, Department of Animal Sensory Physiology, University of Neuchâtel, Switzerland.
This study was financially supported by the School of Public Health, Academic Pivot for Education and Research, Tehran University of Medical Sciences, project No. 85-01-63-3687.
References
- Naddaf SR et al. Molecular characterization of the Anopheles fluviatilis species complex in Iran. Eastern Mediterranean Health Journal, 2003, 9:257–265.
- Sedaghat MM et al. Morphological and molecular characterization of Anopheles (Anopheles) sacharovi Favre, a primary vector of malaria in the Middle East. Systematic Entomology, 2003a, 28:241–256.
- Sedaghat MM et al. The Anopheles maculipennis complex (Diptera: Culicidae) in Iran: molecular characterisation and recognition of a new species. Bulletin of Entomological Research, 2003b, 93:527–535.
- Vatandoost H et al. Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan province, southern Iran. Acta Tropica, 2006b, 97:196–205.
- Omrani Sm et al. Differential responses of Anopheles stephensi (Diptera: Culicidae) to skin emanations of a man, a cow, and a Guinea pig in the olfactometer. Iranian Journal of Arthropod-Borne Diseases, 2010, 4:1–16.
- Kiszewski A et al. A global index representing the stability of malaria transmission. American Journal of Tropical Medicine and Hygiene, 2004, 70:486–498.
- Costantini C et al. Mosquito responses to carbon dioxide in a West African Sudan savanna village. Medical and Veterinary Entomology, 1996, 10:220–227.
- Gillies MT, Wilkes TJ. A comparison of the range of attraction of animal baits and of carbon dioxide for some West african mosquitoes. Bulletin of Entomological Research, 1969, 59:441–458.
- McIver SB, McElliott PE. Effects of release rates on the range of attraction of carbon dioxide to some southwestern Ontario mosquito species. Journal of the American Mosquito Control Association, 1989, 5:6–9.
- Reeves WC. Quantitative field studies on a carbon dioxide chemotropism of mosquitoes. American Journal of Tropical Medicine and Hygiene, 1953, 2:325–331.
- Dekker T et al. L-Lactic acid: a human-signifying host cue for the anthropophilic mosquito Anopheles gambiae. Medical and Veterinary Entomology, 2002, 16:91–98.
- Kline DL et al. Field studies on the potential of butanone, carbon dioxide, honey extract, 1-octen-3-ol, L-lactic and phenols as attractants for mosquitoes. Medical and Veterinary Entomology, 1990, 4:383–391.
- Stryker RG, Young WW. Effectiveness of carbon dioxide and L(+) lactic acid in mosquito light traps with and without light. Mosquito News, 1970, 30:388–393.
- Geier M, Bosch OJ, Boeckh J. Influence of odour plume structure on upwind flight of mosquitoes towards hosts. Journal of Experimental Biology, 1999, 202:1639–1648.
- Omrani S-M et al. Fabrication of an olfactometer for mosquito behavioural studies. Journal of Vector Borne Diseases, 2010, 47:17–25.
- Takken W, Dekker T, Winjholds YG. Odor-mediated flight behaviour of Anopheles gambiae Giles sensu stricto and Anopheles stephensi Liston in response to CO2, acetone and 1-octen-3-ol (Diptera: Culicidae). Journal of Insect Behavior, 1997, 10:395–407.
- Dekker T, Takken W. Differential responses of mosquito sibling species Anopheles arabensis and An. quadriannuatus to carbon dioxide, a man or a calf. Medical and Veterinary Entomology, 1998, 12:136–140.
- Mboera LEG et al. The response of Anopheles gambiae s. l. and A.funestus (Diptera: Culicidae) to tents baited with human odour or carbon dioxide in Tanzania. Bulletin of Entomological Research, 1997, 87:173–178.
- Allan SA, Bernier UR, Kline DL. Laboratory evaluation of avian odors for mosquito (Diptera: Culicidae) attraction. Journal of Medical Entomology, 2006, 43:225–231.
- Mboera LEG, Takken W. Odour-mediated host preference of Culex quinquefasciatus in Tanzania. Entomologia Experimentalis et Applicata, 1999, 92:83–88.
- Davson H, Segal MB. Introduction to physiology. London, Academic Press, 1975:561.
- Kellogg FE, Wright RH. The olfactory guidance of flying insects.III. A technique for observing and recording flight paths. Canadian Entomologist, 1962, 94:486–493.
- Omer SM. Responses of females of Anopheles arabiensis, and Culex fatigans to air currents, carbon dioxide and human hands in a flight-tunnel. Entomologia Experimentalis et Applicata, 1979, 26:142–151.
- Grant AJ, O'Connell RJ. Electrophysiological responses from receptor neurons in mosquito maxillary palp sensilla, In: Bock GR, Cardew G, eds. Ciba Foundation Symposium (200) on Olfaction in Mosquito-Host Interaction. London, John Wiley & Sons, 1996:233–253.
- Kellogg FE. Water vapour and carbon dioxide receptors in Aedes aegypti. Journal of Insect Physiology, 1970, 16:99–108.
- Mboera LEG, Takken W. Carbon dioxide chemotropism in mosquitoes (Diptera: Culicidae) and its potential in vector surveillance and management programmes. Medical and Veterinary Entomology, 1997, 85:355–368.
- Acree F et al. L-Lactic acid: a mosquito attractant isolated from humans. Science, 1968, 161:1346–1347.
- Smallegange RC et al. Synergism between ammonia, lactic acid and carboxylic acids as kiromones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae). Chemical Senses, 2005, 30:145–152.
- Kusakabe Y, Ikeshoji T. Comparative attractancy of physical and chemical stimuli to aedine mosquitoes. Japan Journal of Sanitation Zoology, 1990, 41:219–225.
- Williams CR et al. Geographic variation in attraction to human odor compounds by Aedes aegypti mosquitoes (Diptera: Culicidae): a laboratory study. Journal of Chemical Ecology, 2006b, 32:1625–1634.
- Kostin PV. Reaction of Aedes aegypti (Diptera, Culicidae) to odor of chemical compounds as related to atmospheric pressure. Zoologicheskij Zhurnal, 1983, 6:942–944.
- Steib BM, Geier M, Boeckh J. The effect of lactic acid on odour-related host preference of yellow fever mosquitoes. Chemical Senses, 2001, 26:523–528.
- Murphy MW et al. Attraction of Anopheles (Diptera: Culicidae) to volatile chemicals in Western Kenya. Journal of Medical Entomology, 2001, 38:242–244.
- Davis EE, Sokolove PG. Lactic acid sensitive receptors on the antennae of mosquito Aedes aegypti. Journal of Comparative Physiology, 1976, 105:43–54.


