Annual report of the Regional Director 2015
Health security and International Health Regulations
The incidence of emerging and re-emerging infectious diseases continues to escalate: half the countries in the Region reported a high incidence of emerging and re-emerging infectious diseases in 2015. WHO responded to an increasing number of outbreaks. These included influenza A(H1N1) pdm09 in Libya, Kuwait and Jordan; avian influenza A (H5N1) in Egypt; MERS-CoV in Saudi Arabia and Jordan; cholera in Iraq and Somalia; hepatitis A in Syrian Arab Republic; dengue fever in Yemen; and unknown viral haemorrhagic fever in Sudan. Strategic, operational and technical support was provided to countries for detection, risk assessment and rapid response to emerging infectious diseases and to prevent international spread of these infections.
In response to a Regional Committee resolution (EM/RC/61/R.2), rapid external assessments were conducted by WHO staff and experts towards the end of 2014 and in early 2015 in 20 (out of 22) countries to assess the capacity to deal with a potential importation of Ebola virus. The major gaps identified related to leadership and coordination, capacities at points of entry, surveillance and response, infection control, laboratory capacity and risk communication (Fig. 3). Following this assessment of preparedness and readiness measures, a 90-day regional action plan was developed and implemented during the first quarter of 2015 to help the countries address the critical gaps identified by WHO in the areas of surveillance and response for prevention, detection and effective containment measures. This work contributed to accelerating the progress in implementation of the core capacity requirements under the International Health Regulations (2005).

Fig 3. Comparison of IHR monitoring assessment results and Ebola assessment results, 2014, for the core capacity of surveillance
Significant public health efforts were mounted to contain the cholera epidemic in Iraq. Surveillance systems were enhanced, the health care workforce was rapidly trained on case management, the operational response was stepped up and oral cholera vaccines were deployed to immunize over 300 000 vulnerable people and to prevent spillover of the outbreak into the hard-to-reach areas.
Work continued with a view to setting up a regional network of expert institutions within the framework of the Global Outbreak Alert and Response Network (GOARN) to respond to outbreaks and other health security threats. The guiding principles and rules of engagement for this network will be finalized and the network activated in 2016. The early warning, alert and response network (EWARN) was expanded in crisis-affected countries such as Iraq, Libya, Syrian Arab Republic and Yemen.
The Region has made preparedness and response to an influenza pandemic a priority. In 2015, actions focused on enhancing the early warning surveillance system, building effective rapid response teams, improving laboratory diagnosis, improving risk communication activities, increasing the availability and use of seasonal influenza vaccine, and developing and implementing plans of action for national capacity strengthening for preparedness and response. Seven countries (Afghanistan, Djibouti, Egypt, Jordan, Lebanon, Morocco, Yemen) received funds from the Pandemic Influenza Preparedness (PIP) Framework partnership to improve capacity for pandemic influenza preparedness and response.
In view of the rapidly expanding threat of MERS-CoV, efforts continued to support the countries to improve public health preparedness measures, especially infection prevention and control measures in the health care environment. The Regional Office organized the 4th international scientific meeting on MERS-CoV in May 2015. These meetings have helped the international scientific community to pinpoint the knowledge gaps on the mode and risk factors for transmission in humans and to identify the essential public health research needed to address such gaps.
Significant progress was made in 2015 in addressing antimicrobial resistance. The regional steering committee met for the first time and outlined an operational framework for implementation of the global action plan on antimicrobial resistance, in collaboration with the Food and Agriculture Organization of the United Nations and World Animal Organization (OIE) and in line with the “One Health” concept.
WHO will continue to strategically support high-risk countries in the areas of surveillance, early detection and response to emerging infectious disease outbreaks. Comprehensive and integrated regional preparedness and response plans and strategies will be developed for managing these outbreaks and other health security threats, with the focus on prevention wherever possible, as well as early detection and response. A concerted effort will also be made to build national capacities to strengthen disease surveillance and response in accordance with the International Health Regulations (2005), including risk communication as an integral part of public health emergency interventions.
The laboratory core capacities required under the International Health Regulations (2005) have not yet been met in about half of the countries owing to insufficient funding and inadequate access, quality of testing, equipment, supplies and workforce competency. WHO continued to provide comprehensive support in strengthening national health laboratory systems and services, with a focus on the required core capacity requirements. WHO, in consultation with national and international stakeholders, developed a draft regional health laboratory strategy 2016–2020. The strategy will guide the efforts of countries towards strengthening national health laboratory systems in a sustainable manner.
Work started on establishing a laboratory network to strengthen laboratory surveillance, detection of and response to emerging dangerous pathogens. To obtain the necessary baseline information for establishing such a network, the epidemiological situation of viral haemorrhagic fevers and emerging dangerous pathogens was analysed, and current capacities and practices in the most advanced health laboratories were mapped and analysed. The next step will be to upgrade the facilities and biosafety/biosecurity practices of the target laboratories capacity-building and mentoring, enrolment in regional external quality assessment schemes and laboratory twinning programmes.
A regional strategy for blood and transfusion services was developed through a consultative process, in collaboration with experts and institutes from within the Region and elsewhere. A project collaboration agreement is being prepared between WHO and the International Federation of Blood Donor Organizations to improve voluntary blood donation and donor care services in the Region.
North-South and South-South technical collaboration with existing regional and global partners was strengthened, and expanded to include academia at global as well as regional levels to support countries in IHR implementation. Cross-border collaboration was strengthened through the establishment of bilateral or multilateral plans to address the deficit in IHR capacities at designated land crossings, a feature compounded by conflict, porous borders, and massive displacement within and across borders in many State Parties.
Compliance with requirements related to notification and reporting, and in responding to WHO verification requests in regard to public health events of potential international concern, continued to improve among IHR national focal points. However, further improvement is still needed through effective intersectoral collaboration to ensure efficient and timely notification of public health events outside the direct purview of the health sector.
WHO continued to monitor progress in IHR implementation and report to the Regional Committee, Executive Board and World Health Assembly through the self-assessment questionnaire submitted by State Parties. The 2015 results indicated a regional implementation level of over 60% under various IHR capacities. However, the reliability and validity of the IHR progress based on self-assessment and self-reporting has increasingly been questioned at all levels of the Organization, as well as by the external stakeholders. In this respect, the Regional Committee adopted a resolution (EM/RC62/R.3) establishing a regional assessment commission (IHR-RAC), comprising regional and global experts to assess implementation of the IHR in the Region and to advise Member States on priority actions to develop and maintain core capacities. The Commission was established and had its first meeting in December 2015 at which its terms of reference and working modalities were discussed. The fourth IHR stakeholders’ meeting was also held in December and introduced State Parties to the work of the Commission and to the new approach to accelerate implementation of the Regulations.
An IHR monitoring and evaluation framework was developed by WHO and a global consultation was organized, in Cairo in January 2016, to harmonize the assessment processes and tools with like-minded initiatives like the Global Health Security Agenda (GHSA), FAO and OIE, in compliance with resolution EM/RC62/R.3. Subsequently, a harmonized joint external evaluation (JEE) process and tool for the implementation of IHR capacities was also developed to complement the annual reporting by State Parties. The JEE process and tool are being finalized with input from all relevant stakeholders. Once finalized, JEE assessments will be conducted on a voluntary basis at State Party level with a view to accurately identifying the gaps and developing and implementing country action plans with clear priorities, to ensure health security for all.
Poliomyelitis eradication
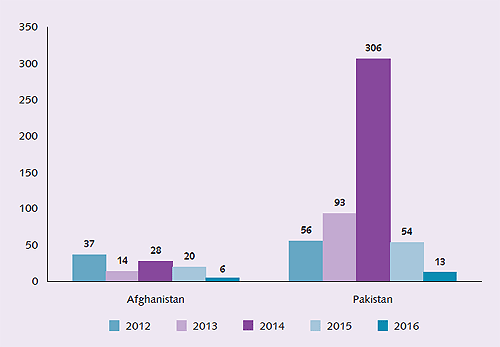
The global progress towards poliomyelitis eradication in 2015 was tremendous (Fig. 4). For the first time in the history of the global initiative, the entire African continent reported no polio cases in more than a year; the date of onset of the most recent case in the continent was 11 August 2014 in Somalia. The only remaining serotype circulating, wild polio virus type 1, was limited to a few areas in only two endemic countries, Pakistan and Afghanistan, both in the Eastern Mediterranean Region. These countries reported a total of 74 cases in 2015, an 80% reduction in case load compared to the number of cases they reported during 2014.
Data as at 17 July 2016
Fig. 4 Decline in cases in polio-endemic countries since 2012
Despite this tremendous progress, as long as wild poliovirus is circulating anywhere there is still a risk of importation for countries in the Region due to extensive population movement and the on-going complex emergency situations in several countries, which have resulted in deteriorating routine immunization coverage. In 2015, 10 polio-free countries of the Region conducted national or sub-national polio immunization campaigns to maintain high levels of immunity and reduce the risk of wild polio virus (WPV) importation or the development of circulating vaccine derived polio virus (cVDPV).
Afghanistan and Pakistan have developed robust national emergency action plans to stop polio transmission in 2016. Both countries made significant progress in reducing transmission through the course of 2015 and in the last 6 months of the year, the traditional high season for poliovirus transmission, only 36 cases were reported in total, the lowest burden of disease ever recorded during this period. In the last quarter of 2015, formal reviews of the multi-country outbreaks in the Middle East and the Horn of Africa that occurred in 2013–2014 concluded that both outbreaks were closed.
A major objective of the polio endgame plan is the withdrawal of oral polio vaccine (OPV) in a phased manner, starting with type 2-containing oral polio vaccine. All countries of the region have successfully switched from trivalent to bivalent oral poliovirus vaccine for routine and campaign use, and have stopped using trivalent oral polio vaccine. It is imperative that all countries of the region fully report on the validated switch process and destroy any remaining oral polio vaccine 2/Sabin2 by 30th July 2016, as part of phase I of the Global Action Plan (GAP III) for poliovirus type 2 containment.
To achieve eradication, the Region must stop the ongoing wild poliovirus transmission in the remaining endemic foci in Afghanistan and Pakistan; maintain population immunity, including in emergency countries and among displaced populations; reach inaccessible children with vaccine; and maintain vigilance and the capacity to detect and respond to any new introduction or outbreak due to wild poliovirus or circulating vaccine-derived virus. In 2016, therefore, focus will continue to be placed on strengthening the capacity of the programmes in Afghanistan and Pakistan through assignment of highly experienced staff to both countries, and on providing strong technical support.
The programmes in Afghanistan, Pakistan and the Horn of Africa will be regularly reviewed through technical advisory group meetings to analyse progress and advise the governments on the most effective technical interventions. Countries at risk will be supported to carry out supplementary immunization activities to maintain high levels of protection, and operational support will be provided to the endemic and at-risk countries to implement planned activities. Regular risk analysis will be conducted to identify risks and develop mitigation strategies. Technical support will be provided for capacity-building in outbreak response and for the development of national preparedness and response plans for polio-free countries. With these activities the objective of the Region is to become polio-free, and stay polio free, in 2016.
HIV, tuberculosis, malaria and tropical diseases
The number of people living with HIV (PLHIV) in the Region is still growing at a fast pace, reaching 330 000 by the end of 2015. Member States have made significant progress in increasing the number of people receiving antiretroviral therapy (ART), from 34 345 in 2014 to 46 345 at the end of 2015. Despite this progress, at 14% the regional ART coverage still remains far from the global target. By the end of 2015, all countries had updated their HIV treatment guidelines according to the latest WHO guidelines. Several countries (Egypt, Islamic Republic of Iran, Lebanon, Morocco, Pakistan/Punjab and Sudan) conducted HIV test-treat-retain cascade analyses which resulted in a deeper understanding of the gaps and lost opportunities in engaging and retaining people living with HIV (PLHIV) in a continuum of HIV testing, care and treatment.
Since the vast majority of PLHIV in the Region do not know their HIV status, much emphasis was placed on initiating an intensified dialogue with national AIDS programme managers and regional civil society networks on innovations in HIV testing policies and service delivery approaches. Implementation of the new WHO consolidated guidelines for HIV testing services was discussed at a regional consultation organized by UNAIDS and WHO and participants identified country-specific priority actions for accelerating uptake of HIV testing. WHO developed and disseminated a training course on HIV basic knowledge and stigma reduction in health care settings. The course was implemented in Morocco and Sudan and is underway in other countries.
Given the high contribution of injecting drug use to the HIV epidemic, a regional review was conducted of access by PLHIV who inject drugs to HIV testing and treatment. The review findings showed that only 6% of PLHIV who inject drugs received HIV treatment in 2014. Those findings were shared and discussed with stakeholders during a regional consultation, conducted in partnership with the Middle East and North Africa Harm Reduction Association (MENAHRA), which resulted in specific recommendations to address barriers to prevention, diagnosis and treatment. Consultations also took place to solicit regional input to the development of new global health sector strategies for HIV, sexually transmitted infections and hepatitis for the period 2016–2021.
Hepatitis is a priority public health problem in the Region, with an estimated 14.8 million and 16 million people chronically infected with hepatitis B and hepatitis C, respectively. New infections in the Region result primarily from unsafe injections and medical procedures. Providing access to well-tolerated and effective medicines and diagnostics is a major challenge for all countries.
To mobilize a coherent public health response that prioritizes effective interventions and promotes equitable access to services, WHO convened and engaged a broad range of stakeholders in the development of regional action plan for viral hepatitis. The regional action plan sets targets in line with the WHO global strategy for viral hepatitis and guides national action plan development. So far, national action plan development was supported in five countries, including Egypt and Pakistan which have the highest burden of hepatitis Cin the Region.
The way forward will place emphasis on universal health coverage, integrated service delivery and adapted service delivery models. Focus on the efficient use of human and financial resources in HIV testing, linkage to care and successful treatment will continue. Concerted efforts will be made to achieve zero tolerance of stigma and discrimination against PLHIV in health care settings. Countries will be supported to develop and implement their national hepatitis plans.
During 2014, slightly more tuberculosis cases (all forms) were notified in the Region compared with 2013 (465 677 and 448 000 respectively). Globally and at the regional level, the main challenge for tuberculosis control continues to be the low case detection rates of all tuberculosis cases and of MDR-TB. In 2014, the case detection rate increased slightly (61 %) compared with 2013 (58%). The treatment success rate was 91%, which is higher than the global target of 85%. Management of multi-drug resistant (MDR) tuberculosis is a key challenge. Out of 15 700 estimated MDR-TB cases, 4348 were confirmed as multi-drug resistant by laboratory test and only 3423 were put on treatment. Limited resources and weak management capacity to deal with multi-drug resistance is a major impediment. Screening of HIV among tuberculosis cases is still limited. In 2014, the HIV status of only 15% of TB patients was known.
The emergency situations in many countries and the widening gap between available resources and need are exposing tuberculosis programmes to bigger threats. Syrian refugees in Jordan and Lebanon are in need of much support, while the health systems are over stretched. This has delayed implementation of the plan for tuberculosis elimination in Jordan. Effective and timely implementation of the national strategic plans for tuberculosis control in Iraq and Yemen is also now impeded by the large numbers of internally displaced persons. A guide for tuberculosis control in complex emergencies was published and a package for management of cross-border tuberculosis and MDR-TB cases was made available. With WHO support, Lebanon and Jordan made successful emergency proposals to the Global Fund to manage tuberculosis among Syrian refugees.
The draft regional strategic plan for tuberculosis for 2016–2020 was developed in consultation with the programme managers. The national tuberculosis programmes were reviewed in six countries and the recommendations of the review missions were subsequently incorporated into the national strategic plans. The Regional Green Light Committee (rGLC) continued its support to ensure effective management of MDR-TB through capacity building, drug resistance surveys, and monitoring and evaluation missions.
Eight countries in the Region have continuous local malaria transmission. Two of these countries (Islamic Republic of Iran and Saudi Arabia) are implementing elimination strategies and are close to reaching the target, with only 187 and 83 local cases, respectively, reported in 2015 (Table 2). Six countries (Afghanistan, Djibouti, Pakistan, Somalia, Sudan and Yemen) have a high malaria burden (Table 3) and face several challenges. WHO estimates that the incidence of malaria in the Region had decreased by 70% in 2015 compared with 2000. Estimated mortality decreased by 64% in the same period. Seven countries achieved the malaria targets set by MDG 6 and resolution WHA58.2, with a reduction of more than 75% in the incidence of microscopically confirmed cases between 2000 and 2014 (Afghanistan, Iraq, Islamic Republic of Iran, Morocco, Oman, Saudi Arabia, Syrian Arab Republic). During the past 15 years the Region achieved great success in reduction of malaria burden. However, in 2014 and 2015 it witnessed outbreaks and an increase in the number of reported cases in some countries. This shows the need for continuing vigilance and investment in malaria control and elimination.
Table 2. Parasitologically-confirmed cases in countries with no or sporadic transmission and countries with low malaria endemicity
Table 2. Parasitologically-confirmed cases in countries with no or sporadic transmission and countries with low malaria endemicity
|
Country |
2013 |
|
2014 |
|
2015 |
|
|
Total reported cases |
Autochthonous |
Total reported cases |
Autochthonous |
Total reported cases |
Autochthonous |
|
|
Bahrain |
182 |
0 |
100 |
0 |
NA |
NA |
|
Egypt |
262 |
0 |
313 |
22 |
291 |
0 |
|
Iraq |
8 |
0 |
2 |
0 |
2 |
0 |
|
Islamic Republic of Iran |
1373 |
519 |
1238 |
376 |
797 |
187 |
|
Jordan |
56 |
0 |
102 |
0 |
59 |
0 |
|
Kuwait |
291 |
0 |
268 |
0 |
309 |
0 |
|
Lebanon |
133 |
0 |
119 |
0 |
125 |
0 |
|
Libya |
340 |
0 |
412 |
0 |
NA |
NA |
|
Morocco |
314 |
0 |
493 |
0 |
510 |
0 |
|
Palestine |
0 |
0 |
NA |
NA |
NA |
NA |
|
Oman |
1451 |
11 |
1001 |
15 |
822 |
4 |
|
Qatar |
728 |
0 |
643 |
0 |
445 |
0 |
|
Saudi Arabia |
2513 |
34 |
2305 |
51 |
2620 |
83 |
|
Syrian Arab Republic |
22 |
0 |
21 |
0 |
12 |
0 |
|
Tunisia |
68 |
4 |
98 |
0 |
88 |
0 |
|
United Arab Emirates |
4380 |
0 |
4575 |
0 |
3685 |
0 |
NA: not available
Table 3. Reported malaria cases in countries with high malaria burden
Table 3. Reported malaria cases in countries with high malaria burden
|
Country |
2013 |
|
2014 |
|
2015 |
|
|
Total reported cases |
Total confirmed |
Total reported cases |
Total confirmed |
Total reported cases |
Total confirmed |
|
|
Afghanistan |
319742 |
46114 |
290079 |
83920 |
350044 |
103377 |
|
Djibouti |
1684 |
1684 |
9439 |
9439 |
NA |
NA |
|
Pakistan |
3472727 |
281755 |
3666257 |
270156 |
3776244 |
202013 |
|
Somalia |
9135 |
7407 |
26174 |
11001 |
NA |
NA |
|
Sudan |
989946 |
592383 |
1207771 |
1068506 |
NA |
NA |
|
Yemena |
149451 |
102778 |
122812 |
86707 |
96348 |
68938 |
NA: not available
a The estimated reporting completeness in 2015 was 47% due to the situation
The Regional Committee endorsed the regional malaria action plan 2016–2020 for implementation of the Global technical strategy for malaria 2016–2030. Health Assembly A regional integrated vector management strategy 2016–2020 was developed in consultation with key experts and Member States.
A new online reporting system using DHIS2 was used to support malaria surveillance. Entomological surveillance, including insecticide resistance monitoring in priority countries and drug efficacy monitoring in malaria endemic countries, was also supported. The regional training course on quality assurance of malaria diagnosis was conducted in collaboration with Gezira University, Sudan. The Regional Office has completed the implementation of the WHO-UNEP-GEF demonstration projects on sustainable alternatives to DDT. The evidence generated and the capacities developed during the projects will contribute to updating strategies for integrated vector management and strengthening vector control activities in the Region.
Significant scale up of mass treatment of schistosomiasis with WHO-donated praziquantel took place in Sudan thanks to an innovative mechanism enabling domestic funding, as well as to international partnerships. Priority endemic areas in Yemen were also targeted early in the year. Planning for mapping in Somalia was finalized, and funds mobilized, following the establishment of a neglected tropical disease programme in the Ministry of Health. Support was provided to three countries (Egypt, Iraq, Oman) to plan and implement surveys aimed at confirming interruption of transmission in view of the initiation of the WHO’s verification process for elimination of schistosomiasis.
In 2015, more countries applied for WHO-donated albendazole and mebendazole to treat preschool and school-age children for soil-transmitted helminthiasis, compared with 2014, including Afghanistan, Iraq, Somalia and Syrian Arab Republic. WHO’s collaboration with UNRWA was strengthened to provide free medicines to schoolchildren in the Agency’s five fields of operation (Jordan, Lebanon, Palestine (Gaza Strip and West Bank) and Syrian Arab Republic).
The final steps towards elimination of lymphatic filariasis as a public health problem were taken in Egypt and Yemen. Sudan completed mapping and finalized operational planning to scale-up mass treatment with WHO-donated albendazole and ivermectin in 2016. Elimination of onchocerciasis was demonstrated in one focus in Sudan and actions were taken to achieve the same goal in the three remaining ones. In Yemen, a pilot survey to delimitate the onchocerciasis endemic area was carried out, funds for treatment were mobilized through partners, and planning for mass treatment was finalized.
With regard to leishmaniasis, WHO continued to contribute to provision of case management to all affected country programmes: Afghanistan, Iraq and Syrian Arab Republic, Somalia and Sudan.
In 2014, 213 899 new leprosy cases were detected. Elimination as a public health problem (less than 1 prevalent case per 10 000 population) has been reached at national level in all countries of the Region. However, five countries (Egypt, Pakistan, Somalia, Sudan and Yemen,) still have pockets of intense transmission, and need to strengthen case-detection activities. A steady decline in the proportion of grade 2 disabilities among newly-detected cases has been observed in recent years, confirming that cases are being detected at progressively earlier stages. Multidrug therapy for leprosy was provided by WHO to all requesting countries.
Trachoma mapping was completed in Sudan, is ongoing in Egypt, Pakistan and Yemen, and was planned for Afghanistan and Somalia. Treatment activities with azithromycin and tetracycline, and other components of the SAFE strategy (surgery, facial cleanliness and environmental improvements) were scaled up in Pakistan and Sudan.
Sudan is the only country in the Region which remains to be certified free from dracunculiasis. No cases have been reported since 2014 and the country is in pre-certification. Field activities aimed at assessing readiness to undergo the certification process were implemented in 2015.
Immunization and vaccines
The regional average of DTP3 coverage was estimated at 80% in 2015. While 14 countries have maintained the target achievement of ≥90% coverage, in Syrian Arab Republic it dropped to 41% in 2015 (WHO–UNICEF estimates). An estimated 3.3 million children missed DTP3 immunization in 2015, 94% of whom were in countries facing difficult situations: Afghanistan, Iraq, Pakistan, Somalia, Sudan, Syrian Arab Republic and Yemen.
Eight countries have achieved ≥95% coverage with the first dose of measles-containing vaccine (MCV1), and 21 countries provided the routine second dose of measles vaccine with variable levels of coverage. Eight countries reported very low incidence of measles (fewer than 5 cases per million population), four of which continued to achieve zero incidence and are ready for verification of elimination. Jordan restored its measles-free status following a major outbreak with incidence in 2013.
With regard to new vaccines, Yemen introduced rubella vaccine into its routine immunization and Sudan implemented the second phase of a yellow fever campaign. Inactivated polio vaccine (IPV) was introduced in nine countries where it was not previously part of routine immunization. As a result, IPV is now in use in all countries of the Region except Egypt which was not supplied with IPV vaccine because of the global shortage. The Region completed the switch from using trivalent (tOPV) to bivalent (bOPV) oral polio vaccine in routine immunization by mid May 2016.
The national immunization programmes continued to face several challenges in 2015, including complications related to vaccine delivery to conflict-affected areas, procurement and management systems and stock-out of several vaccines. Support was provided to countries with low routine immunization coverage, including intensifying outreach activities, implementation of acceleration campaigns to increase coverage and sustaining cold chain and vaccine management capacity. Support was also provided for development and implementation of national plans to reach unvaccinated and under-vaccinated populations
In Somalia support was provided in implementing a coverage improvement plan, human resource capacity building and developing a comprehensive multi-year plan (cMYP). In the Syrian Arab Republic, support was provided for a comprehensive programme review, development of cMYP, assessment of vaccine management and capacity-building on vaccine management. Iraq conducted programme reviews at the governorate level and implemented plans to vaccinate refugees, internally displaced persons and hard-to-reach children, especially in inaccessible areas. Huge support was provided to Yemen to maintain the immunization programme, including implementation of five rounds of intensified outreach activities to districts with low coverage or that were hard to reach and strengthening and maintenance of the cold chain. Five countries assessed the status of measles elimination and nine countries implemented supplementary measles immunization at national or subnational levels.
Support was provided to countries for improvement of immunization data management systems and data quality, for accreditation of national measles/rubella laboratories, and capacity-building in effective vaccine management. WHO continued to support and monitor the regional network for measles/rubella case-based surveillance. Support continued to be provided also to the regional surveillance network of bacterial meningitis, bacterial pneumonia and rotavirus. This included provision of laboratory supplies, capacity-building, monitoring and evaluation of performance and coordinating the external laboratory quality control system.
The Eastern Mediterranean vaccine action plan (EMVAP) was endorsed by the Regional Committee as a framework for implementation of the global vaccine action plan (GVAP). WHO will continue to provide the necessary technical support and mobilization of resources for updating, implementation, monitoring and evaluation of cMYP and annual plans of action, with special focus on countries and areas with low vaccination coverage.
Six countries continued to achieve the target of a functional national regulatory authority WHO is supporting countries to strengthen the required regulatory functions, particularly the registration of vaccines such as IPV and bOPV as part of the polio endgame strategy, as well as to strengthen the implementation of a quality management system for some national regulatory authorities in countries that are supported by the Pandemic Influenza Preparedness (PIP) Framework partnership in order to improve regulatory capacity for pandemic influenza preparedness and response.
Significant progress continued to be made in vaccine safety, and the regional pharmacovigilance network was launched in a regional meeting on pharmacovigilance held in September 2015. Achieving a functioning national regulatory authority is inhibited in some countries by a number of challenges, including lack of clear vision, staff turnover and lack of financial resources to allow the authorities to perform their duties independently, as recommended by WHO.
[1] For tuberculosis case detection, WHO receives data a year later, thus case detection data relate to 2014 and treatment outcome data to 2015.


