Secondary analysis of Turkish national data investigating negative birth outcomes and stunting related to social factors and adolescent motherhood
Ceren V. Akpinar1, Asli A. Teneler2
1Department of Public Health, Giresun University Faculty of Medicine, Giresun, Turkey. 2Giresun Central Community Health Centre, Giresun, Turkey (Correspondence: A.A. Teneler:
Abstract
Background: Adolescent motherhood can cause lifelong health inequalities for mothers and children.
Am: To compare the frequency of negative birth outcomes and stunting in children aged 5 years of adolescent and nonadolescent mothers, and to determine the relationship with sociodemographic factors based on the Turkey Demographic and Health Survey.
Methods: This was a secondary analysis of the Turkey Demographic and Health Survey 2018. Logistic regression analysis was conducted on a sample of 2755 women aged 15–49 years who gave a live birth in the past 5 years.
Results: Term low birthweight and stunting were significantly higher in children of adolescent mothers. Multivariable analysis revealed that lack of education, poverty, and living in eastern Turkey increased the risk of delivering a term low birthweight infant. The risk of being stunted was 2.22 times higher in women with low socioeconomic status, and 2.86 times higher in low birthweight infants.
Conclusion: A large sample from the Turkey Demographic and Health Survey emphasized the necessity of planning of maternal and child health services by considering maternal education level, income inequality, and even regional inequality. These results support the notion that macroenvironmental factors have a marked impact on maternal and child health.
Keywords: adolescent pregnancy, low birthweight, stunting, social inequalities, Turkey
Citation: Akpinar CV, Teneler AA. Secondary analysis of Turkish national data investigating negative birth outcomes and stunting related to social factors and adolescent motherhood. East Mediterr Health J. https://doi.org/10.26719/emhj.23.074 Received: 16/8/2022; Accepted: 22/12/2022
Copyright: © Authors; licensee World Health Organization. EMHJ is an open access journal. All papers published in EMHJ are available under the Creative Commons Attribution Non-Commercial ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Introduction
Adolescent pregnancy can result in lifetime health disparities for mothers and their children. Compared with nonadolescent mothers, adolescent mothers are more likely to have lower educational level, less financial independence, worse mental health, and less social support (1–4). All these factors may contribute to the high prevalence of malnutrition in adolescent mothers (5). Globally, 13% of all births are given by women aged 15–19 years in emerging countries (6). In Turkey, the adolescent pregnancy rate was 10.2% in 1993 but it decreased to 4% by 2018 (7). Although the rate of adolescent pregnancy has decreased over the years, it is still important when the resultant health and social problems are considered.
The most important indicator of chronic malnutrition in children is stunting (8). In the last 20 years, although there has been a decrease globally in stunting in children aged < 5 years, differences between regions and within countries remain (9,10). In Turkey, the rate of stunting in children aged 20 million births per year (12). In Turkey, 12% of live births are low birthweight (7). Socioeconomic factors, maternal age, maternal education, maternal body mass index, and antenatal care and nutrition are risk factors for low birthweight (3, 4, 13, 14). As expected, adolescent mothers are at higher risk of adverse birth outcomes, such as premature birth and low birthweight, because they usually have worse antenatal care and conditions (4, 15, 16).
Although there are data on the socioeconomic vulnerabilities and health risks of adolescent mothers in Turkey, it is unclear what effect adolescent motherhood has on negative birth outcomes and stunting. We used the national data from the 2018 Turkey Demographic and Health Survey to: (1) compare the frequency of negative birth outcomes (low birthweight, preterm birth, and term low birthweight) and stunting in children aged < 5 years of adolescent and nonadolescent mothers; and (2) investigate the sociodemographic factors affecting negative birth outcomes and stunting in children of adolescent and nonadolescent mothers.
Methods
Study design, participants and measurements
This study used secondary data analysis of the 2018 Turkey Demographic and Health Survey that focused on adolescent and nonadolescent mothers aged 15–49 years and their children aged 0–5 years. The survey was a nationally representative cross-section that was conducted by Hacettepe University Institute of Population Studies every 5 years since 1993 to monitor population health and maternal and child health indicators. The purpose of the survey was to gather data at the household level to formulate national indicators related to demographics, fertility, child mortality, maternal health, and nutritional status of women and children. The survey data are used by public institutions, especially the Ministry of Health, for the planning of health services (17).
For the main data of the 2018 Turkey Demographic and Health Survey, a weighted, multistage, stratified cluster sampling approach was used. Two questionnaires for households and individuals were used to collect data by face-to-face interview. Out of 9056 women aged 15–49 years in these households, 7346 (81.1%) were interviewed. The sampling design details and the results of the main data of the study were reported in the 2018 Turkey Demographic and Health Survey Analysis and Report (7).
We used the individual questionnaire dataset to analyse 2755 women aged 15–49 years who had a live birth up to 5 years before the questionnaire was administered. There were no available data for the birthweight and date of birth of 117 children, or for the height of 653 children. Identification of the secondary dataset and the inclusion and exclusion criteria for the study are shown in Figure 1.
The dependent variables were having a child in the past 5 years with negative birth outcomes (low birthweight, preterm birth, and term low birthweight) and stunting. Stunting was defined using the 2006 WHO growth standard reference point based on z score ≤ 2 standard deviations (18). Negative birth outcomes were: (1) low birthweight, < 2500 g at birth; (2) preterm, born before 37 weeks’ gestation; and (3) term low birthweight, born at ≥ 37 weeks’ gestation and < 2500 g at birth.
Maternal age was the main independent variable and was categorized as adolescent (15–19 years) and nonadolescent (20–29, 30–39, and 40–49 years). Maternal educational level was defined as no education, primary, secondary, and high school, and while evaluating the variable, the secondary and high school categories were combined. Welfare status was categorized as richest, richer, middle, poorer, and poorest according to wealth index, and evaluated by combining them into 3 groups as rich (richest and richer), middle, and poor (poorer and poorest). The country data were divided into 5 regions of west, south, central, north, and east. The residential area was considered as urban or rural. The gender of the child was categorized as male or female. For the stunting dependent variable, children’s age was evaluated monthly and categorized into 3 groups (0–11, 12–23, and 24–59 months). Antenatal visits were categorized as yes (mother attended ≥ 4 visits) or no (mother did not attend ≥ 4 visits or did not know the answer). The variables used in this study were categorized according to the Demographic and Health Surveys guidelines (19).
Ethical considerations
The 2018 Turkey Demographic and Health Survey was evaluated and approved by Hacettepe University Ethics Commission. The researchers obtained permission on 2 December 2021 from Hacettepe University Institute of Population Studies to use the data.
Data analysis
Results
In the study group of 2755 mothers, 189 (6.8%) were adolescent. Table 1 shows the sociodemographic characteristics of adolescent and nonadolescent mothers. Eighty-two (43.4%) adolescent mothers had secondary or higher education compared with 1309 (51.0%) nonadolescent mothers. One hundred and twenty-five (66.1%) adolescent mothers and 1233 (48.1%) nonadolescent mothers were poorer or poorest. Sixty-six (34.9%) adolescent and 768 (29.9%) nonadolescent mothers lived in rural areas. One hundred and six (56.0%) adolescent and 218 (28.1%) nonadolescent mothers had not received adequate antenatal care. There were significant differences between adolescent and nonadolescent mothers in terms of educational level, welfare status, and antenatal care during pregnancy (P < 0.05).
Table 2 compares the rates of negative birth outcomes and the indicators of chronic malnutrition according to maternal age (adolescent/nonadolescent category). There were 2638 negative birth outcomes: 338 (12.8%) low birthweight, 405 (15.4%) preterm birth, and 205 (7.8%) term low birthweight. In addition, 116 (5.5%) children were stunted. The rate of low birthweight was 14.8% in adolescent mothers and 12.7% in nonadolescent mothers. Preterm birth rate was similar for the 2 groups. The rate of delivering a term low birthweight infant was 11.8% in adolescent mothers and 7.5% in nonadolescent mothers, and this difference was significant (P < 0.05). Stunting, which is an indicator of chronic malnutrition in children aged < 5 years, was seen in 8.7% of children of adolescent mothers and 5.2% of children of nonadolescent mothers, and this difference was significant (P < 0.05).
Table 3 shows the factors associated with term low birthweight in a sample of 2638 women who gave birth in the past 5 years. Adolescent motherhood was evaluated as the main independent variable. Term low birthweight, which was significantly different in the children of adolescent and nonadolescent mothers, lost significance in the adjusted analysis (AOR 1.50, 95% CI: 0.90–2.49). Multivariate analysis showed that lack of maternal education (AOR 1.75, 95% CI: 1.22–2.50), poverty (AOR 2.09, 95% CI: 1.44–3.02), and living in eastern Turkey (AOR 1.39, 95% CI: 1.01–1.92) increased the risk of term low birthweight.
Multivariate analysis of the factors associated with stunting in 2102 women who gave birth in the last 5 years is shown in Table 4. Adolescent motherhood was evaluated as the main independent variable. There was a significant difference in child stunting between adolescent and nonadolescent mothers but after adjustment, there was no significance (AOR 0.77, 95% CI: 0.36–1.64). The risk of being stunted was 2.22 times higher (95% CI: 1.47–3.50) in the poorest/poorer group compared with other socioeconomic status. Children born with low birthweight were 2.86 times more likely to be stunted than children born with normal birthweight (95% CI: 1.90–4.30). The risk of stunting was increased 1.69 times (95% CI: 1.12–2.53) in children aged 12–23 months compared with 0–11 months, and 1.65 times (95% CI: 1.20–2.65) in children aged 24–59 months.
Discussion
We evaluated the sociodemographic characteristics of adolescent and nonadolescent mothers, negative birth outcomes, and frequency of stunting of their children in Turkey using data from the 2018 Turkey Demographic and Health Survey. We also investigated the risk factors associated with term low birthweight and stunting. We found that adolescent mothers had lower educational level, were poorer, and did not receive adequate antenatal care compared with nonadolescent mothers. Although term low birthweight and stunting were risk factors in children of adolescent compared with nonadolescent mothers, they were no longer significant when adjusted for socioeconomic variables. This indicates that if socioeconomic status of adolescent mothers is improved, their risk of negative birth outcomes and stunting will be reduced. Our results could be explained by women with good socioeconomic status not becoming pregnant during adolescence. However, some studies have reported that young maternal age is not the sole reason for negative birth outcomes, and other factors such as emotional response, coping skills, and social resources may be involved (20, 21). If adolescent pregnancies are well planned and there is adequate prenatal care, they will cease to be high risk (22).
Low maternal educational level was a risk factor for term low birthweight in multivariate analysis, which is consistent with previous studies (23, 24). Term low birthweight was higher in the poorest households and in eastern Turkey, which is less well developed than western Turkey. The significant association between low socioeconomic status and low birthweight that was shown in this study was also found in other studies (25, 26). Low socioeconomic and educational statuses lead to low health consciousness and low nutritional status, which can increase the risk of low birthweight. There is considerable variation in the prevalence of low birthweight across regions and within countries; however, most occurs in low- and middle-income countries and especially in the most vulnerable populations in these countries.
Multivariate analysis showed that being poorer was a risk factor for stunting. Similarly, in the National Family Health Survey of India, stunting was more common in those living in poorer rural areas (27). These findings were similar to a study conducted in the United Republic of Tanzania and indicated that children in lower socioeconomic groups had a greater risk of stunting compared with those in higher socioeconomic groups (28). Several other studies have found that household socioeconomic status is a prominent predictor of child stunting (29, 30). The availability of high-quality foods and affordability of nutrient-rich foods affect a family’s ability to provide a healthy diet and prevent child stunting. Higher household income enables more to be spent on food and child care (12). This is in line with the suggestion that households with larger income, as a proxy for household wealth, have more money to spend on child nutrition, which lowers the prevalence of stunting (31).
Our large sample data from the 2018 Turkey Demographic and Health Survey indicated that economic status was the main risk factor for negative birth outcomes and stunting. Special attention must be paid to individuals who have low educational levels, low income, and live in eastern Turkey. To improve child health, we suggest that it is more important to improve education and reduce inter-regional poverty rather than trying to reduce adolescent pregnancies. These results support the idea that macroenvironmental factors have a marked impact on maternal and child health.
In our multivariate analysis, being older than 2 years was a risk factor for stunting. Children’s age in months had a nonlinear, upward-sloping effect on the probability of stunting. Therefore, children tend to be more stunted as they age, although this effect diminishes over time (31). Stunting was more common in children older than 18 months in the National Family Health Survey of India, and children aged < 24 years had a higher risk of stunting in Rwanda (27, 32). The results are consistent with the theory that worsening intrauterine conditions, as measured by birth size and other factors, increase the likelihood of stunting (31). The fact that the risk of stunting increases with age indicates that chronic malnutrition is becoming severe.
Our study had some limitations. Firstly, there were some missing data, such as age at which the children ceased breastfeeding, accompanied by many confounding factors, maternal body mass index, and maternal birth weight. Secondly, the main data in the 2108 Turkey Demographic and Health Survey were collected 4 years ago. Finally, the lack of anthropological data for all children led to a reduction in the sample size. Regardless of the limitations, it was possible to identify the risk factors for stunting and term low birthweight in the eastern and western provinces of Turkey, after multivariate linear regression analysis or adjustment for all missing confounders.
Conclusion
This study adds to the limited research examining the association between adolescent pregnancy and adverse birth outcomes and stunting in Turkey. Our results suggest that it would be more beneficial to make changes at the macroenvironmental level to reduce low birthweight and stunting. The social role of women living in eastern Turkey is perhaps the root cause of these health inequalities. Strengthening the position of women in society will prevent adolescent pregnancies and contribute to the prevention of other health inequalities. Our results emphasize the necessity of planning maternal and child health services by considering the educational level of women, income inequalities, and even regional inequalities, while defining the risk factors related to adolescent pregnancy.
References
1. Boden JM, Fergusson DM, John Horwood L. Early motherhood and subsequent life outcomes. J Child Psychol Psychiatry. 2008 Feb;49(2):151–60. https://doi.org/10.1111/j.1469-7610.2007.01830.x PMID:18093114
2. Norris S, Norris ML, Sibbald E, Aubry T, Harrison ME, Lafontaine G et al. Demographic characteristics associated with pregnant and postpartum youth referred for mental health services in a community outreach center. J Can Acad Child Adolesc Psychiatry. 2016 Fall;25(3):152–8. PMID:27924145
3. Le Roux K, Christodoulou J, Stansert-Katzen L, Dippenaar E, Laurenzi C, M. le Roux I et al. A longitudinal cohort study of rural adolescent vs adult South African mothers and their children from birth to 24 months. BMC Pregnancy Childbirth. 2019; 19(24):1–8. https://doi.org/10.1186/s12884-018-2164-8
4. Banke-Thomas OE, Banke-Thomas AO, Ameh CA. Factors influencing utilisation of maternal health services by adolescent mothers in low-and middle-income countries: a systematic review. BMC Pregnancy Childbirth. 2017;17(65):1–14. https://doi.org/10.1186/s12884-017-1246-3
5. Olodu MD, Adeyemi AG, Olowookere SA, Esimai OA. Nutritional status of under-five children born to teenage mothers in an urban setting, south-western Nigeria. BMC Res Notes. 2019 Mar 4;12(1):116. https://doi.org/10.1186/s13104-019-4147-x PMID:30832719
6. Darroch JE, Woog V, Bankole A. Adding it up: costs and benefits of meeting the contraceptive needs of adolescents. New York: Guttmacher Institute, 2016 (https://www.guttmacher.org/report/adding-it-meeting-contraceptive-needs-of-adolescents, accessed 15 April 2023).
7. Turkey Demographic and Health Survey 2018. Ankara: Hacettepe University Institute of Population Studies, T.R. Presidency of Turkey Directorate of Strategy and Budget, and TÜBITAK; 2019 ( https://fs.hacettepe.edu.tr/hips/dosyalar/Ara%C5%9Ft%C4%B1rmalar%20-%20raporlar/2018%20TNSA/TDHS2018_mainReport_compressed.pdf, accessed 15 April 2023).
8. Stunting in a nutshell [website]. Geneva: World Health Organization; 2015 (https://www.who.int/news/item/19-11-2015-stunting-in-a-nutshell, accessed 15 April 2023).
9. Joint child malnutrition estimates [website]. Geneva: World Health Organization (https://www.who.int/data/gho/data/themes/topics/joint-child-malnutrition-estimates-unicef-who-wb, accessed 15 April 2023).
10. Malnutrition in children [website]. UNICEF (https://data.unicef.org/topic/nutrition/malnutrition/, accessed 15 April 2023).
11. Ndemwa M, Wanyua S, Kaneko S, Karama M, Anselimo M. Nutritional status and association of demographic characteristics with malnutrition among children less than 24 months in Kwale County, Kenya. Pan Afr Med J. 2017 Nov;28:265. https://doi.org/10.11604/pamj.2017.28.265.12703 PMID:29881508
12. Global nutrition targets 2025: low birth weight policy brief. Geneva: World Health Organization; 2014 (https://apps.who.int/iris/handle/10665/149020, accessed 15 April 2023).
13. Mumbare SS, Maindarkar G, Darade R. Maternal risk factors associated with term low birth weight neonates: a matched-pair case control study. Indian Pediatr. 2012 Jan;49(1):25-28. https://doi.org/10.1007/s13312-012-0010-z PMID:21719926
14. Nabugoomu J, Seruwagi GK, Corbett K, Kanyesigye E, Horton S, Hanning R. Needs and barriers of teen mothers in rural Eastern Uganda: stakeholders’ perceptions regarding maternal/child nutrition and health. Int J Environ Res Public Health. 2018 Dec 7;15(12):2776. https://doi.org/10.3390/ijerph15122776 PMID:30544550
15. Amjad S, MacDonald I, Chambers T, Osornio‐Vargas A, Chandra S, Voaklander D et al. Social determinants of health and adverse maternal and birth outcomes in adolescent pregnancies: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2019 Jan;33(1):88-99. https://doi.org/10.1111/ppe.12529 PMID:30516287
16. Chen XK, Wen SW, Fleming N, Demissie K, Rhoads GG, Walker M. Teenage pregnancy and adverse birth outcomes: a large population based retrospective cohort study. Int J Epidemiol. 2007 Apr;36(2):368–73. https://doi.org/10.1093/ije/dyl284 PMID:17213208
17. Turkey Demographic and Health Survey 2018 – final report. Ankara: Hacettepe University Institute of Population Studies, T.R. Presidency of Turkey Directorate of Strategy and Budget, and TÜBITAK; 2019 (https://dhsprogram.com/publications/publication-FR372-DHS-Final-Reports.cfm, accessed 15 April 2023).
18. WHO child growth standards : training course on child growth assessment. Geneva: World Health Organization; 2008 (https://apps.who.int/iris/handle/10665/43601, accessed 15 April 2023).
19. The DHS Program Demographic and Health Surveys. Available datasets [website]. Rockville, MD, USAID (https://dhsprogram.com/data/available-datasets.cfm, accessed 15 April 2023).
20. Klein JD. American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005 Jul;116(1):281–6. https://doi.org/ 10.1542/peds.2005-0999 PMID:15995071
21. Aruda MM, Waddicor K, Frese L, Cole JCM, Burke P. Early pregnancy in adolescents: diagnosis, assessment, options counseling, and referral. J Pediatr Health Care. 2010 Jan–Feb;24(1):4–13. https://doi.org/10.1016/j.pedhc.2008.11.003 PMID:20122473
22. Geist RR, Beyth Y, Shashar D, Beller U, Samueloff A. Perinatal outcome of teenage pregnancies in a selected group of patients. J Adolesc Gynecol. 2006 Jun;19(3):189–93. https://doi.org/10.1016/j.jpag.2006.02.005 PMID:16731412
23. Jafari F, Eftekhar H, Pourreza A, Mousavi J. Socio-economic and medical determinants of low birth weight in Iran: 20 years after establishment of a primary healthcare network. Public Health. 2010 Mar;124(3):153–8. https://doi.org/10.1016/j.puhe.2010.02.003 PMID:20226486
24. Chen Y, Li G, Ruan Y, Zou L, Wang X, Zhang W. An epidemiological survey on low birth weight infants in China and analysis of outcomes of full-term low birth weight infants. BMC Pregnancy Childbirth. 2013 Dec 26;13:242–50. PMID:24370213
25. Viengsakhone L, Yoshida Y, Harun-Or-Rashid M, Sakamoto J. Factors affecting low birth weight at four central hospitals in vientiane, Lao PDR. Nagoya J Med Sci. 2010 Feb;72(1-2):51-58. https://doi.org/10.1186/1471-2393-13-242 PMID:20229703
26. Sharma M, Kumar D, Huria A, Gupta P. Maternal risk factors of low birth weight in Chandigarh India. Internet J Health. 2008;9(1):1–4. https://doi.org/10.5580/10f1
27. National Family Health Survey (NFHS-3) 2005–06 India. Volume 1. Mumbai: International Institute of Population Sciences; 2007 (https://dhsprogram.com/pubs/pdf/frind3/frind3-vol1andvol2.pdf, accessed 15 April 2023).
28. Mtongwa RH, Festo C, Elisaria E. A comparative analysis of determinants of low birth weight and stunting among under five children of adolescent and non-adolescent mothers using 2015/16 Tanzania Demographic and Health Survey (TDHS). BMC Nutr. 2021 Nov 4;7(1):1–10. https://doi.org/10.1186/s40795-021-00468-6 PMID:34732260
29. Bukusuba J, Kaaya AN, Atukwase A. Predictors of stunting in Children Aged 6 to 59 months: a case-control study in Southwest Uganda. Food Nutr Bull. 2017 Dec;38(4):542–53. https://doi.org/10.1177/0379572117731666 PMID:28978233
30. Wamani H, Astrøm AN, Peterson S, Tumwine JK, Tylleskär T. Boys are more stunted than girls in sub-Saharan Africa: a meta-analysis of 16 demographic and health surveys. BMC Pediatr. 2007 Apr 10;7:17). https://doi.org/10.1186/1471-2431-7-17 PMID:17425787
31. Van Der Knaap I, The determinants of sex differences in child stunting in Sub Saharan Africa: a multilevel logistic regression analysis [thesis]. Nijmegen: Radboud University; 2018.
32. Habimana S, Biracyaza E. Risk factors of stunting among children under 5 years of age in the eastern and western provinces of Rwanda: analysis of Rwanda Demographic and Health Survey 2014/2015. Pediatric Health Med Ther. 2019 Oct 25;10:115–30. https://doi.org/10.2147/PHMT.S222198 PMID:31695558
Table 1. Sociodemographic characteristics of adolescent and nonadolescent mothers (N= 2755)
|
Sociodemographic characteristics |
Adolescent mother, n (%) |
Nonadolescent mother, n (%) |
Total n (%) |
P |
|
Educational level None Primary Secondary or higher |
27 (14.3) 80 (42.3) 82 (43.4) |
437 (17.0) 820 (32.0) 1309 (51.0) |
464 (16.8) 900 (32.7) 1391 (50.5) |
0.01 |
|
Welfare status Poorest/poorer Middle Richer/richest |
125 (66.1) 34 (18.0) 30 (15.9) |
1233 (48.1) 507 (19.8) 826 (32.1) |
1358 (49.3) 541 (19.6) 856 (31.1) |
<0.01 |
|
Region West South Central North East |
45 (23.8) 32 (16.9) 34 (18.0) 12 (6.3) 66 (34.9) |
646 (25.2) 348 (13.6) 437 (17.0) 233 (9.1) 902 (35.2) |
691 (25.1) 380 (13.8) 471 (17.1) 245 (8.9) 968 (35.1 ) |
0.53 |
|
Residential area Urban Rural |
123 (65.1) 66 (34.9) |
1798 (70.1) 768 (29.9) |
1921 (69.7) 834 (30.3) |
0.08 |
|
Antenatal visit (≥ 4 visits)a Yes No |
83 (43.9) 106 (56.1) |
1844 (71.9) 218 (28.1) |
1927 (85.6) 324 (14.4) |
<0.01 |
|
aSome missing data. |
||||
Table 2. Number and percentage distribution of negative birth outcomes and stunting of children by maternal age
|
|
Adolescent mother, n (%) |
Non |
Total n (%) |
P |
|
Negative birth outcomes (N = 2638: 169 adolescent and 2469 nonadolescent mothers) |
||||
|
Low birthweight |
25 (14.8) |
313 (12.7) |
338 (12.8) |
0.24 |
|
Preterm birth |
28 (1 |
377 (15.3 |
405 (1 |
0.51 |
|
Term low birthweight |
20 (11.8) |
185 (7.5) |
205 (7.8) |
0.04 |
|
Chronic malnutrition (N = 2102: 138 adolescent and 1964 nonadolescent mothers) |
||||
|
Stunting |
12 (8.7) |
104 (5.3 |
116 (5.5) |
0.03 |
Table 3. Logistic regression results for the factors associated with term low birthweight
|
|
|
N |
n (%) |
Crude OR |
95% CI |
P |
AOR |
95% CI |
P |
||
|
Maternal age, yr |
15–19 |
169 |
20 (11.8) |
1.65 |
1.01 |
2.70 |
0.04 |
1.50 |
0.90 |
2.49 |
0.11 |
|
20–49 (ref) |
2469 |
185 (7.5) |
|
|
|
|
|
|
|
|
|
|
Educational level |
None |
407 |
64 (15.7) |
2.76 |
2.01 |
3.79 |
<0.01 |
1.75 |
1.22 |
2.50 |
0.02 |
|
Primary |
856 |
70 (8.2) |
1.08 |
0.80 |
1.46 |
0.5 |
|
|
|
|
|
|
Secondary and higher (ref) |
1375 |
71 (5.2) |
|
|
|
|
|
|
|
|
|
|
Welfare status
|
Poorest/poorer |
1262 |
145 (11.5) |
2.84 |
2.08 |
3.88 |
<0.01 |
2.09 |
1.44 |
3.02 |
<0.01 |
|
Middle |
531 |
34 (6.4) |
0.77 |
0.52 |
1.13 |
0.18 |
|
|
|
|
|
|
Rich/richest (ref) |
845 |
26 (3.1) |
|
|
|
|
|
|
|
|
|
|
Region |
West (ref) |
673 |
10 (4.5) |
|
|
|
|
|
|
|
|
|
South |
368 |
31 (8.4) |
1.10 |
0.74 |
1.65 |
0.61 |
|
|
|
|
|
|
Central |
464 |
22 (4.7) |
0.54 |
0.34 |
1.15 |
0.08 |
|
|
|
|
|
|
North |
241 |
11 (4.6) |
0.54 |
0.29 |
1.01 |
0.05 |
|
|
|
|
|
|
East |
892 |
104 (11.7) |
2.15 |
1.61 |
2.86 |
<0.01 |
1.39 |
1.01 |
1.92 |
0.04 |
|
|
Residential area |
Urban (ref) |
1856 |
126 (6.8) |
|
|
|
|
|
|
|
|
|
Rural |
782 |
79 (10.1) |
1.54 |
1.14 |
2.07 |
0.004 |
0.96 |
0.69 |
1.33 |
0.83 |
|
|
Antenatal visit |
Yes (ref) |
1886 |
125 (6.6) |
|
|
|
|
|
|
|
|
|
No |
752 |
80 (10.6) |
2.04 |
1.30 |
3.20 |
0.001 |
1.21 |
0.89 |
1.65 |
0.21 |
|
|
Child sex |
Male (ref) |
1335 |
92 (6.9) |
|
|
|
|
|
|
|
|
|
Female |
1303 |
113 (8.7) |
1.28 |
0.96 |
1.70 |
0.08 |
|
|
|
|
|
AOR = adjusted odds ratio; CI = confidence interval.
Table 4. Logistic regression results for the factors associated with stunting
|
|
|
N |
n (%) |
Crude OR |
95% CI |
P |
AOR |
95% CI |
P |
||
|
Maternal age, yr |
15–19 |
138 |
12 (8.7) |
1.23 |
1.02 |
2.60 |
0.03 |
0.77 |
0.36 |
1.64 |
0.50 |
|
20–49 (ref) |
1964 |
104 (5.2) |
|
|
|
|
|
|
|
|
|
|
Educational level |
None |
350 |
42 (12.0) |
1.94 |
1.33 |
2.82 |
<0.01 |
1.01 |
0.63 |
1.61 |
0.96 |
|
Primary |
706 |
52 (7.4) |
0.97 |
0.69 |
1.38 |
0.08 |
|
|
|
|
|
|
Secondary and higher (ref) |
1046 |
63 (6.0) |
|
|
|
|
|
|
|
|
|
|
Welfare status |
Poorest/poorer |
1070 |
114 (10.7) |
2.74 |
1.91 |
3.93 |
<0.01 |
2.22 |
1.47 |
3.50 |
<0.01 |
|
Middle |
405 |
21 (5.2) |
0.62 |
0.39 |
1.07 |
0.06 |
|
|
|
|
|
|
Rich/richest (ref) |
627 |
22 (3.5) |
|
|
|
|
|
|
|
|
|
|
Region |
West (ref) |
528 |
26 (4.9) |
|
|
|
|
|
|
|
|
|
South |
308 |
24 (7.8) |
1.05 |
0.67 |
1.65 |
0.81 |
|
|
|
|
|
|
Central |
343 |
20 (5.8) |
0.73 |
0.45 |
1.19 |
0.20 |
|
|
|
|
|
|
North |
208 |
16 (7.7) |
1.03 |
0.60 |
1.77 |
0.89 |
|
|
|
|
|
|
East |
715 |
71 (9.9) |
1.66 |
1.20 |
2.31 |
0.02 |
1.08 |
0.73 |
1.60 |
0.68 |
|
|
Residential area |
Urban (ref) |
1461 |
95 (6.5) |
|
|
|
|
|
|
|
|
|
Rural |
641 |
62 (9.7) |
1.54 |
1.10 |
2.15 |
0.01 |
0.99 |
0.67 |
1.48 |
0.99 |
|
|
Birthweight |
<2500 |
248 |
38 (15.3) |
3.01 |
2.01 |
4.48 |
<0.01 |
2.86 |
1.90 |
4.30 |
<0.01 |
|
≥2500 (ref) |
1781 |
101 (5.7) |
|
|
|
|
|
|
|
|
|
|
Child sex |
Male |
1072 |
82 (7.6) |
|
|
|
|
|
|
|
|
|
Female (ref) |
1030 |
75 (7.3) |
0.94 |
0.68 |
1.31 |
0.74 |
|
|
|
|
|
|
Child age, mo |
0–11 (ref) |
401 |
14 (3.5) |
|
|
|
|
|
|
|
|
|
12–23 |
380 |
39 (10.3) |
1.55 |
1.06 |
2.27 |
0.02 |
1.69 |
1.12 |
2.53 |
0.01 |
|
|
24–59 |
1321 |
104 (7.9) |
1.17 |
1.11 |
1.65 |
0.03 |
1.65 |
1.20 |
2.65 |
0.02 |
|
AOR = adjusted odds ratio; CI = confidence interval.
Figure 1. Identification of secondary dataset.
Cigarette use and exposure to second-hand smoke and advertising in Tunisian adolescents, 2001 to 2017
Yosr Ayedi,1 Chahida Hariz,1 Afef Skhiri1 and Radhouane Fakhfakh1
1Department of Epidemiology and Biostatistics, Abderrahmane Mami Hospital, Ariana, Tunisia. (Correspondence to Yosr Ayedi:
Abstract
Background: The Global Youth Tobacco Survey was conducted in Tunisia in 2001, 2007, 2010 and 2017.
Aims: To describe the trends in cigarette use among Tunisian adolescents and their exposure to second-hand smoke and tobacco advertising from 2001 to 2017.
Methods: The Global Youth Tobacco Survey is a school-based cross-sectional survey conducted by the World Health Organization. It uses a two-stage cluster sampling design to obtain a representative sample of students aged 13–15 years. A standardized questionnaire is used for data collection. The prevalence and 95% confidence intervals (CI) of ever and current cigarette use, exposure to second-hand smoke in and outside the home, and exposure to tobacco advertising were compared over the 4 years.
Results: Current cigarette use decreased from 11.1% (95% CI: 10.0–12.3%) in 2001 to 7.7% (95% CI: 6.5–9.0%) in 2017, P < 0.001. Exposure to second-hand smoke at home decreased from 62.5% (95% CI: 60.7–64.2%) to 46.7% (95% CI: 44.5–49.0%) over the same period, P < 0.001, but exposure outside the home increased from 65.4% (95% CI: 63.7–67.1%) in 2001 to 73.3% (95% CI: 71.2–75.3%) in 2017, P < 0.001. Exposure to anti-tobacco messages in the media fell from 87.8% (95% CI: 86.3–89.1%) in 2001 to 64.4% (95% CI: 62.2–66.5%) in 2017, P < 0.001.
Conclusion: While the prevalence of cigarette use and second-hand smoke exposure at home fell, exposure outside the home increased. Efforts are needed to ensure compliance with smoke-free laws to decrease the prevalence of second-hand smoke.
Keywords: tobacco use, tobacco smoke pollution, adolescent, prevalence, Tunisia.
Citation: Ayedi Y; Hariz C; Skhiri A; Fakhfakh R. Cigarette use and exposure to second-hand smoke and advertising in Tunisian adolescents, 2001 to 2017. East Mediterr Health J. https://doi.org/10.26719/emhj.23.075 Received:09/05/2022; accepted: 04/01/2023
Copyright © World Health Organization (WHO) 2023. Some rights reserved. This work is available under the CC BY-NC-SA 3.0 IGO license https://creativecommons.org/licenses/by-nc-sa/3.0/igo
Introduction
Tobacco was the main cause of death in males in 2019 worldwide, responsible for 20% of deaths in males. For women, tobacco was the sixth leading cause of death worldwide, responsible 15.4% of all deaths in women (1). Worldwide, in 2019, about 1.14 billion people aged 15 years and older smoked cigarettes (2). Generally, regular adult smokers began smoking during adolescence and one third started at 14 years (3). People who smoke their first cigarette before the age of 18 years are more likely to become heavy smokers and nicotine dependent in the future, and are less likely to quit, which puts them at higher risk of lung cancer or other tobacco-induced diseases (4).Considerable effort has been made globally to control tobacco use by helping smokers to quit and preventing smoking initiation.
In 2004, 603 000 deaths were estimated to be related to second-hand smoke; 28% of these death occurred in children (6). The increase in people’s knowledge of the effects of tobacco use and second-hand smoke as a result of the media and anti-tobacco messages has helped tobacco control efforts (7). The World Health Organization (WHO) launched the Framework Convention on Tobacco Control (FCTC) in 2003, which was the first international treaty on tobacco control (8). In line with the FCTC, WHO introduced the WHO MPOWER measures: M for Monitoring tobacco use and prevention policies, P for Protecting people from tobacco smoke; O for Offering help to quit tobacco use; W for Warning about the dangers of tobacco; E for Enforcing bans on tobacco advertising, promotion and sponsorship; and R for Raising taxes on tobacco.
Tunisia started a national strategic plan to curb the epidemic of tobacco use in adults and young people in 1998. The strategy was further enforced by Tunisia’s ratification of the FCTC in 2010 (10). In Tunisia, the prevalence of smoking among adult males was reported to be 48.3% (95% confidence interval (CI): 46.3–50.3%) according to the Tunisian Health Examination Survey in 2016 (9). The Global Youth Tobacco Survey (GYTS) is a main component of the MPOWER plan of action. It is a multinational survey conducted by WHO (11) in more than 185 countries to monitor tobacco use among young people aged 13–15 years (12). In Tunisia, this survey has been conducted four times: in 2001, 2007, 2010 and 2017. To our knowledge, the GYTS is the only national survey that examined exposure to second-hand smoke and to the media and advertising in young people.
The aim of our study was to identify the trends in cigarette use in Tunisian adolescents from 2001 to 2017 and to describe their exposure to second-hand smoke and the media and advertising related to tobacco.
Methods
Study design
The GYTS is a cross-sectional, descriptive and school-based survey conducted by WHO. It uses a two-stage cluster sample design to obtain representative samples of students aged 13–15 years. In Tunisia, the age 13–15 years old matches students in the seventh, eighth and ninth school grades. In the GYTS, the complete list of all public schools is sent to the tobacco centre at the United States Centers for Disease Control and Prevention (CDC) where schools are chosen randomly in proportion to the number of students enrolled in the specified grade. Then, classes are randomly chosen according to the city population and size (one or two classes per school).
The GYTS in Tunisia are carried out in April and May of each survey year. Physicians and nurses of medical schools are responsible for data collection, under the direction of the entity Medicine School and University, which takes care of the health of students in schools and universities. The surveys are funded by WHO. Each student in the age range 13–15 years range (seventh, eighth and ninth grades) in the selected classes who is present in the class on the day of survey is eligible to participate in the study.
Questionnaire
The GYTS survey uses a standard methodology and the questionnaire was validated by CDC and WHO experts (13). It contains core questions about the main tobacco concerns focusing on:
• prevalence of all smoked tobacco products and conventional cigarettes
• smokers’ access to tobacco products
• smokers’ behaviours related to stopping smoking
• exposure to the media and advertising
• exposure to second-hand smoke.
The questionnaire has been translated into Arabic and then re-translated into English and sent back to CDC for further checks to ensure accuracy and reliability. It was first pretested with a focus group of adolescents to endure the translation was pertinent and precise. The questionnaire contained 69 questions in 2001, 63 questions in 2007, 70 questions in 2010 and 63 in 2017: 27 questions are common to all four surveys.
We focused on trends in the prevalence of conventional cigarette smoking and exposure to second-hand smoke and to the media and advertising.
Measures
Ever cigarette smoker was defined as someone who had ever smoked cigarettes, even if they had only taken one or two puffs in their lives. Current cigarette user was defined as someone who had smoked cigarettes anytime during the past 30 days, that is, had given any answer other than 0 days to the question, “In the past 30 days, how many days did you smoke cigarettes?”
Participants were considered to have been exposed to second-hand smoke inside the home if they gave any answer other than 0 days to the question, “In the past 7 days, how many days have people smoked in your home, in your presence?” Similarly, they were considered exposed outside the home if they gave any answer other than 0 days to the question and “In the past 7 days, how many days have people smoked in your presence in places other than in your home?”
Consent
Oral consent of the parents of the students is taken the day before the survey.
Data analysis
Anonymized data were available at the official CDC site (https://nccd.cdc.gov/GTSS/rdPage.aspx?rdReport=OSH_GTSS.ExploreByLocation&rdRequestForwarding=Form). We analysed the data using R version 4.2.0 and R studio version 2022.07.01 software. In each survey, adjusted and weighting factors were applied to each student record to adjust for the probability of selection and non-response (by school, class and student).
The weighting factor was: W = W1 × W2 × F1 × F2 × F3 × F4, where: W1 = the reverse of probability of selection of the school; W2 = the reverse of probability of selection of the class within the school; F1 = adjustment factor of non-response of schools according to size (large, medium, small); F2 = adjustment factor of class calculated by school; F3 = adjustment factor of student non-response calculated within this class; and F4 = adjustment factor post-stratification calculated by sex and grade.
The weighting factor was applied through the survey package of R Studio. Unweighted numbers of students were inserted in tables. Indicators were described using weighted percentages reflecting the population estimates. We calculated the 95% confidence intervals (CI) for each proportion. The association between two qualitative variables was assessed with the chi-squared test. Trends were assessed using the Cochrane Armitage trend test. A two-sided 5% significance level was used for all calculations.
Results
From 2001 to 2017, the number of schools included in the survey increased from 50 to 67. The overall response rate varied from 94.1% (2942/3127) in 2001 to 92.9% (1863/2005) in 2017 (Table 1).
Conventional cigarettes
The male to female ratio was about the same in the 4 years: 0.97 in 2001 and 0.93 in 2017. In 2001, about 23.0% (95% CI: 21.5–24.5%) of the respondents had tried to smoke a cigarette, even if only one or two puffs: 35.4% (95% CI: 32.9–37.9%) of boys and 11.4% (95% CI: 9.9–13.1%) of girls. This proportion increased to 25.0% (95% CI: 23.1–27.1%) in 2017, with the increase greater in boys: 38.8% (95% CI: 35.6–42.0%) in boys and 11.6% (95% CI: 9.6–13.8%) in girls. However, these increases were not significant (P > 0.05).
As for current cigarette use, the prevalence decreased significantly from 11.1% (95% CI: 10.0–12.3%) in 2001 to 7.7% (95% CI: 6.5–9.0%) in 2017 (P < 0.001). In boys over the same period, the prevalence of smoking decreased from 19.1% (95% CI: 17.1–21.2%) to 14.2% (95% CI: 12.1–16.7%; P < 0.001). In girls, the prevalence decreased from 3.6% (95% CI: 2.8–4.7%) to 1.4% (95% CI: 0.8–2.4%; P < 0.001) (Table 2).
Exposure to second-hand smoke
Between 2001 and 2017, exposure to second-hand smoke at home in the 7 days before the survey decreased significantly from 62.5% (95% CI: 60.7–64.2%) to 46.7% (95% CI: 44.5–49.0%; P < 0.001). This reduction was significant for both boys and girls (P < 0.001) (Table 3).
Exposure to second-hand smoke outside the home increased significantly between 2001 and 2017, from 65.4% (95% CI: 63.7–67.1%) to 73.3% (95% CI: 71.2–75.3%; P < 0.001). This exposure increased significantly for both boys and girls (P < 0.001) (Table 3).
Most respondents were in favour of implementing smoke-free places by law, although this support fell significantly from 87.0% (95% CI: 85.7–88.2%) in 2001 to 81.8% (95% CI: 80.0–83.5%) in 2017 (P < 0.001), and decreased for both boys and girls (P < 0.001) (Table 3)
Exposure to the media and advertising
Exposure to anti-tobacco messages in the media deceased from 87.8% (95% CI: 86.3–89.1%) in 2001 to 64.4% (95% CI: 62.2–66.5%) in 2017 (P < 0.001). This exposure decreased significantly for both boys and girls (P < 0.001) (Table 4). However, exposure to anti-tobacco messages at sports and cultural events increased significantly, from 34.2% (95% CI: 32.5–35.9%) in 2001 to 72.2% (95% CI: 70.1–74.2%) in 2017 (P < 0.001). This exposure increased significantly for both boys and girls (P < 0.001) (Table 4). Two thirds of students (67.3%; 95% CI: 64.7–69.8%) had seen advertising for tobacco use in 2010. This proportion fell significantly to 43.7% (95% CI: 41.2–46.2%) in 2017 (P < 0.001). This exposure decreased significantly for both boys and girls (P < 0.001) (Table 4). The proportion of respondents who had had received free promotional cigarettes was small and did not change significantly over the years (Table 4).
Discussion
The GYTS is one of the most important tobacco monitoring tools and helps countries implement the MPOWER package. The questions are in line with the MPOWER package and focus on important aspects of tobacco use and tobacco control. Monitoring the prevalence of tobacco use over time is essential to identify changes and link the national tobacco control strategy to the current situation.
Conventional cigarettes
One in four students had ever tried to smoke a cigarette: one boy out of three and one girl out of 10. In the United States, data from the National Youth Tobacco Survey from 2014 to 2016 showed that 21% of adolescents had ever tried to smoke a cigarette (14). The GYTS in the United Arab of Emirates in 2013 focused on expatriate adolescents only and reported that 32% of boys had tried to smoke a cigarette, at a mean age of 12–13 years (15). A previous Tunisian national survey, which included 4172 adolescents aged 12–20 years from public and private schools, reported that among students aged 12–14 years, 26.9% had tried to smoke a cigarette in their lives (16). In the Sfax region in the south of Tunisia, ever cigarette smoking was reported in 16.7% of school students (32.6% of boys and 5.9% of girls) (17). Our findings are similar to these studies and indicate a high prevalence of cigarette experimentation among boys and girls in Tunisia.
Our findings show that the prevalence of current cigarette use in adolescent Tunisians has decreased over time, overall and for boys and girls. According to the last Youth Risk Behavior Survey in the United States conducted in 2019, a significant decrease in current cigarette use had occurred among students in the ninth grade (14–15 years), from 13.5% in 2009 (18) to 3.8% in 2019 (19). In a 45-country analysis of GYTS data in 2013 and 2014, the median global prevalence of current cigarette use across all countries was 6.8% (9.7% in boys and 3.5% in girls), which is lower than the prevalence in our four surveys overall and for boys, but higher than current cigarette use we found for girls. Given the findings of the 2017 GYTS in Tunisia, the country has the fourth highest prevalence of adolescent cigarette use in the Middle East and North African region, after Jordan (2014 GYTS), Lebanon (2013 GYTS) and Qatar (2013 GYTS) (20). Other studies of North African countries showed that a greater proportion of Tunisian boys smoked than Egyptian, Libyan, Moroccan and Sudanese boys (21). In Malaysia, the prevalence of current cigarette use decreased from 19.9% in 2003 to 14.8% in 2016, which is almost double of the prevalence in our study (22). In Morocco, Tunisia’s neighbour, the current cigarette use among 13–15-year-old schoolchildren increased from 3.0% in 2006 to 5.2% in 2010, but both are lower than the prevalence in Tunisian schoolchildren (23). In the city of Sousse in Tunisia, the results of a cross-sectional survey in 2013–2014 in 16 public schools found that 4.5% of participants were cigarette users, which is lower than the national prevalence in the GYTS surveys (24). Even though cigarette consumption in Tunisian schoolchildren fell from 2001 to 2017, it is nonetheless still high and needs to be tackled to reduce the its prevalence further.
Exposure to second-hand smoke
Second-hand smoke outside the home in adolescents increased from 2001 to 2017. A study in 131 countries found that exposure to second-hand smoke outside the home was 57.6% in 2018 and it had not decreased from 1999 – it remained the same in 46 of 131 countries (35.1%) and increased in 40 (30.5%). This increase was found in almost all WHO regions (exposure was 59.4% for exposure at least one day a week in the Middle East and North Africa region) and in countries that did not ratify the FCTC (25). The overall exposure to second-hand smoke in public places among non-smoking adolescents was 44.2% across 168 countries from 1999 to 2008. The exposure was higher in boys than girls. Exposure ranged from 39.8% in the Middle East and North Africa region to 73.7% in the European region (26). In Africa, from 2006 to 2011, exposure to second-hand smoke among adolescents was 39.0%; it ranged from 24.9% in Cape Verde to 80.4% in Mali, with no differences between the sexes (27). These results show that exposure to second-hand smoke in Tunisia is among the highest in the world.
The proportion of students in favour of laws that establish smoke-free places decreased for both sexes. Tunisia established its first tobacco law (no. 98-17) in February 1998 which aimed to protect people from tobacco harm. Article 10 of this law prohibits smoking in public places (28). This law was enforced by the decree of November 1998 (29), decree of September 2009 (30) and ratification of the FCTC in 2010. Article 8 of the FCTC calls for countries to adopt and implement effective national legislations to protect people from exposure to tobacco in indoor and outdoor public places (8). However, the compliance of Tunisians and respect of these laws seem to be weak given the high rates of exposure to second-hand smoke outside the home (31). In the most recent report of MPOWER in the Middle East and North Africa region, Tunisia had a score of 1 out of 3 for smoke-free places, which means only up to two public places were completely smoke free (32). A longitudinal study found evidence that, in addition to positive impact on exposure to second-hand smoke, laws on smoke-free places led to a possible decrease in smoking prevalence (33).
Exposure to the media and advertising
In the 2001 Tunisian GYTS, a greater proportion of respondents were exposed to anti-tobacco messages in the media (internet, magazines, television) than respondents in the 2017 GYTS. This result is similar to findings in Greece (34), Italy (35) and Myanmar (36). A longitudinal study in the United States found a positive effect of anti-tobacco messages on teenagers’ susceptibility to smoke. In fact, this exposure decreased the susceptibility to smoke by 2 or 3 years (37). From 2010 to 2017, the proportions of students exposed to cigarette advertising at points of sale decreased. A systematic review in 2009 concluded that exposure to promotion of cigarette use at points of sale increased the odds of ever smoking, frequent smoking or occasional smoking (38). This explains why the tobacco industry spends around 80% of their advertising budget on promotions at points of sale (39). Article 13 of the FCTC calls for countries to ban every kind of tobacco promotions, advertisings and sponsorships. In Tunisia, law no. 98-17 forbids all types of promotion of tobacco products in public places, but it does not include a ban on promotion at points of sale (28).
Strengths and limitations
A strength of our study is that the GYTS is the only standardized worldwide survey on tobacco use and attitudes in adolescents aged 13–15 years. In addition, the GYTS is a national survey conducted in all governorates and cities in the country. Furthermore, the sample size of students who answered the questionnaire was large and the response rates were always more than 92%.
Our study has some limitations. Smoking behaviour and exposure to second-hand smoke were self-reported and no quantitative method was used to confirm the students’ responses, which may introduce biases. In addition, only students in public schools were included, thus students in private schools or adolescents who were not in school were not represented. Students in private schools and teenagers who don’t go to schools represent about 10% of Tunisian adolescents according to a 2015 report (40).
Conclusions
WHO recommends that countries implement a monitoring survey every 5 years. It has been 5 years since the last GYTS in Tunisia and a new GYTS survey is needed. In addition, efforts to ensure complete compliance with smoke-free laws are needed to decrease the prevalence of second-hand smoke. Finally, a complete ban of point of sales promotions is strongly recommended to decrease the exposure of vulnerable young people to this tobacco advertising.
Acknowledgements
This study was a collaborative project of WHO, CDC and the Ministry of Health of Tunisia. We thank the study participants and research assistants.
Funding: None.
Competing interests: None declared.
References
1. Abbafati C, Abbas KM, Abbasi-Kangevari M, Abd-Allah F, Abdelalim A, Abdollahi M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–49. https://doi.org/10.1016/S0140-6736(20)30752-2
2. Reitsma MB, Kendrick PJ, Ababneh E, Abbafati C, Abbasi-Kangevari M, Abdoli A, et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397(10292):2337–60. https://doi.org/10.1016/S0140-6736(21)01169-7
3. The epidemiology of tobacco use among young people in the United States and worldwide. In: Preventing tobacco use among youth and young adults: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2012.
4. Cantrell J, Bennett M, Mowery P, Xiao H, Rath J, Hair E, et al. Patterns in first and daily cigarette initiation among youth and young adults from 2002 to 2015. PLoS One. 2018;13(8):1–20. https://doi.org/10.1371/journal.pone.0200827
5. Hughes J, Kabir Z, Bennett K, Hotchkiss JW, Kee F, Leyland AH, et al. Modelling future coronary heart disease mortality to 2030 in the British Isles. PLoS One. 2015;10(9):1–12. https://doi.org/ 10.1371/journal.pone.0138044
6. Öberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377(9760):139–46. https://doi.org/10.1016/S0140-6736(10)61388-8
7. Blake KD, Viswanath K, Blendon RJ, Vallone D. The role of tobacco-specific media exposure, knowledge, and smoking status on selected attitudes toward tobacco control. Nicotine Tob Res. 2010;12(2):117–26. https://doi.org/10.1093/ntr/ntp184
8. WHO Framework Convention on Tobacco Control [Internet]. Geneva: World Health Organization; 2005 (https://fctc.who.int/, accessed 20 January 2022).
9. Tunisian Health Examination Survey – 2016. Tunis: République Tunisienne, Ministère de la Santé, Institut National de la Santé; 2019 (http://www.santetunisie.rns.tn/images/rapport-final-enquete2020.pdf, accessed 20 January 2022).
10. Harizi C, El-Awa F, Ghedira H, Audera-Lopez C, Fakhfakh R. Implementation of the WHO Framework Convention on Tobacco Control in Tunisia: Progress and challenges. Tob Prev Cessat. 2020;6:1–8. https://doi.org/10.18332/tpc/130476
11. Global Youth Tobacco Survey. Tunisia [Internet]. Geneva: World Health Organization (https://extranet.who.int/ncdsmicrodata/index.php/catalog/GYTS#_r=&collection=&country=217&dtype=&from=1999&page=1&ps=&sid=&sk=&sort_by=nation&sort_order=&to=2019&topic=&view=s&vk=, accessed 9 September 2020).
12. Global youth tobacco survey [Internet]. Geneva: World Health Organization (https://www.who.int/tobacco/surveillance/gyts/en/, accessed 31 August 2020).
13. Global Youth Tobacco Survey Collaborative Group. Global youth tobacco survey (GYTS): core questionnaire with optional questions. Version 1.2. Atlanta, GA: Centers for Disease Control and Prevention; 2014.
14. Sharapova S, Reyes-Guzman C, Singh T, Phillips E, Marynak KL, Agaku I. Age of tobacco use initiation and association with current use and nicotine dependence among US middle and high school students, 2014–2016. Tob Control. 2020;29(1):49–54. https://doi.org/10.1136/tobaccocontrol-2018-054593
15. Asfour LW, Stanley ZD, Weitzman M, Sherman SE. Uncovering risky behaviors of expatriate teenagers in the United Arab Emirates: a survey of tobacco use, nutrition and physical activity habits. BMC Public Health. 2015;15(1):944. https://doi.org/10.1186/s12889-015-2261-9
16. Fakhfakh R, Jaidane I, Hsairi M, Ben Hamida AM. Les facteurs de risque et de protection de l’initiation à la cigarette chez les adolescents tunisiens [Cigarette smoking initiation among Tunisian adolescents: Risk and protective factors]. Rev Epidemiol Sante Publique. 2015;63(6):369–79. http://dx.doi.org/10.1016/j.respe.2015.09.005
17. Ben Ayed H, Yaich S, Ben Hmida M, Ben Jemaa M, Trigui M, Karray R, et al. Prevalence and factors associated with smoking among Tunisian secondary school-adolescents. Int J Adolesc Med Health. 2021;33(6):379–87. https://doi.org/10.1515/ijamh-2019-0088
18. Centers for Disease Control and Prevention. Youth risk behavior surveillance—United States, 2009. Surveillance summaries, June 2010. MMWR Surveill Summ. 2010;59(5):1–142.
19. Creamer MLR, Everett Jones S, Gentzke AS, Jamal A, King BA. Tobacco product use among high school students – youth risk behavior survey, United States, 2019. MMWR Suppl. 2020;69(1):56–63. https://doi.org/10.15585/mmwr.su6901a7
20. D’Angelo D, Ahluwalia IB, Pun E, Yin S, Palipudi K, Mbulo L. Current cigarette smoking, access, and purchases from retail outlets among students aged 13–15 years—Global Youth Tobacco Survey, 45 countries, 2013 and 2014. MMWR Morb Mortal Wkly Rep. 2016;65(34):898–901. https://doi.org/10.15585/mmwr.mm6534a3
21. Madkour AS, Ledford EC, Andersen L, Johnson CC. Tobacco advertising/promotions and adolescents’ smoking risk in Northern Africa. Tob Control. 2014;23(3):244–52. https://doi.org/10.1136/tobaccocontrol-2012-050593
22. Lim K, Ghazali S, Lim H, Kee C, Cheah Y, Pradhaman Singh B, et al. Tobacco use and other aspects related to smoking among school-going adolescents aged 13–15 years in Malaysia: analysis of three cross-sectional nationally representative surveys in 2003, 2009 and 2016. Tob Induc Dis. 2020;18(September):1–10. https://doi.org/10.18332/tid/127231
23. Shaikh MA. Prevalence, correlates, and changes in tobacco use between 2006 and 2010 among 13–15 year Moroccan school attending adolescents. J Pak Med Assoc. 2014;64(11):1306–9.
24. Nawel Z, Jihen M, Rim G, Sana B, Hassen G. Tobacco use: the main predictor of illicit substances use among young adolescents in Sousse, Tunisia. Int J Adolesc Med Health. 2018;32(5). https://doi.org/10.1515/ijamh-2017-0213
25. Ma C, Heiland EG, Li Z, Zhao M, Liang Y, Xi B. Global trends in the prevalence of secondhand smoke exposure among adolescents aged 12–16 years from 1999 to 2018: an analysis of repeated cross-sectional surveys. Lancet Glob Health. 2021;9(12):e1667–78. https://doi.org/10.1016/S2214-109X(21)00365-X
26. Ma C, Heiland EG, Li Z, Zhao M, Liang Y, et al. Secondhand smoke exposure among never-smoking youth in 168 countries. J Adolesc Health. 2015;56(2):167–73. https://doi.org/10.1016/S2214-109X(21)00365-X
27. Owusu D, Mamudu HM, John RM, Ibrahim A, Ouma AEO, Veeranki SP. Never-smoking adolescents’ exposure to secondhand smoke in Africa. Am J Prev Med. 2016;51(6):983–98. https://doi.org/10.1016/j.amepre.2016.08.040
28. [Law no. 98-17 of February 23 1998, relative to prevention of the harmful effects of smoking]. Journal Officiel du la République Tunisienne;1998 27 février:399–400.
29. Decree no. 98–2248, November, 16 1998. Fixant les lieux affectés à l'usage collectif dans lesquels il est interdit de fumer [Identifying smoke-free public places]. Tunis: Government of Tunisia; 1998.
30. Décret no. 2009-2611 du 14 septembre 2009, complétant le décret n° 98-2248 du 16 novembre 1998 fixant les lieux affectés à l’usage collectif dans lesquels il est interdit de fumer [Completing decree no. 98-2248 of November, 16 1998]. Tunis: Government of Tunisia; 2009.
31. Ben Amar W, Chakroun A, Zribi M, Khemekhem Z, Ben Jemaa F, Maatoug S. Dispositif législatif de lutte anti-tabagique en Tunisie : entre insuffisances et défaut d’application [Anti tobacco legislation and regulation in Tunisia: between shortcomings and lack of application]. JIM Sfax. 2017;17:21–6.
32. Heydari G, Zaatari G, Al-Lawati JA, El-Awa F, Fouad H. MPOWER, needs and challenges: trends in the implementation of the WHO FCTC in the Eastern Mediterranean Region. East Mediterr Health J. 2018;24(01):63–71. https://doi.org/10.26719/2018.24.1.63
33. Becker CM, Lee JGL, Hudson S, Hoover J, Civils D. A 14-year longitudinal study of the impact of clean indoor air legislation on state smoking prevalence, USA, 1997–2010. Prev Med (Baltim). 2017;99:63–6. https://doi.org/10.1016/j.ypmed.2017.01.016
34. Kyrlesi A, Soteriades ES, Warren CW, Kremastinou J, Papastergiou P, Jones NR, et al. Tobacco use among students aged 13–15 years in Greece: the GYTS project. BMC Public Health. 2007;7(1):3. https://doi.org/10.1186/1471-2458-7-3
35. Gorini G, Gallus S, Carreras G, Cortini B, Vannacci V, Charrier L, et al. A long way to go: 20-year trends from multiple surveillance systems show a still huge use of tobacco in minors in Italy. Eur J Public Health. 2019;29(1):164–9. https://doi.org/10.1093/EURPUB/CKY132
36. Tun N, Chittin T, Agarwal N, New M, Thaung Y, Phyo P. Tobacco use among young adolescents in Myanmar: findings from global youth tobacco survey. Indian J Public Health. 2017;61(5):54. https://doi.org/10.4103/ijph.IJPH_236_17
37. Weiss JW, Cen S, Schuster D, Unger J, Johnson CA, Mouttapa M, et al. Longitudinal effects of pro‐tobacco and anti‐tobacco messages on adolescent smoking susceptibility. Nicotine Tob Res. 2006;8(3):455–65. https://doi.org/10.1080/14622200600670454
38. Paynter J, Edwards R. The impact of tobacco promotion at the point of sale: a systematic review. Nicotine Tob Res. 2009;11(1):25–35. https://doi.org/10.1093/ntr/ntn002
39. Ma H, Reimold AE, Ribisl KM. Trends in cigarette marketing expenditures, 1975–2019: an analysis of federal trade commission cigarette reports. Nicotine Tob Res. 2022;24(6):919–23. https://doi.org/10.1093/ntr/ntab272
40. Middle East and North Africa out-of-school children initiative. Summary, Tunisia: country report on out-of-school children. Amman: UNICEF MENA Regional Office; 2015 (https://www.unicef.org/mena/media/6661/file/Tunisia%20Country%20Report%20on%20OOSC%20Summary_EN.pdf%20.pdf, accessed 20 January 2022).
Table 1. Schools, classes and students in included in the Global
Table 2. Ever and current cigarette smoking, by sex and year, Tunisia Global Youth Tobacco Survey
Table 3. Exposure to second-hand smoke and perceptions of mandated smoke-free places, by year, Tunisia Global Youth Tobacco Survey
Table 4. Prevalence of exposure to the media and advertising, by year, Tunisia, Global Youth Tobacco Survey
Response to cholera outbreaks, Somalia, 2017–2019: challenges, interventions and lessons learnt
Mutaawe Lubogo,1 Buliva Evans,2 Abubakar Abdinasir,2 Elnossery Sherein,2 Tayyab Muhammad,2 Ahmed M. Mohamed,3 Aden Hussein,3 Fayez Abdulrazeq2 and Malik Sk Md Mamunur1
1World Health Organization, Somalia Country Office, Mogadishu, Somalia.
2Emergency Programme, World Health Organization Regional Office for the Eastern Mediterranean, Cairo, Egypt.
3Federal Ministry of Health, Mogadishu, Somalia. (Correspondence to Mutaawe Lubogo:
Abstract
Background: Somalia reported repeated cholera outbreaks between 2017 and 2019. These outbreaks were attributed to the presence and occurrence of multiple risk factors for cholera, which made response challenging.
Aims: To describe the challenges faced by Somalia in responding to cholera outbreaks between 2017 and 2019 and provide lessons for Somalia and other countries with a similar context on how to better prepare for future outbreaks.
Methods: We reviewed outbreak response reports, surveillance records and preparedness plans for cholera outbreaks in Somalia from January 2017 to December 2019 and other relevant literature. We present data on cholera-related response indicators including cholera cases and deaths and case fatality rates in the 3 years. Qualitative data were collected from five focus group discussions and 10 key informant interviews to identify challenges, interventions and lessons learnt from the Somali experience.
Results: In 2017, 78 701 cholera cases and 1163 related deaths were reported (case fatality rate 1.48%), in 2018, 6448 cholera cases and 45 deaths were reported (case fatality rate 0.70%), while in 2019, 3089 cases and four deaths were reported in Somalia (case fatality rate 0.13%). The protracted conflict, limited access to primary health care, and limited access to safe water and proper sanitation among displaced populations were the main drivers of repeated cholera outbreaks.
Conclusions: Periodic assessment of response to and preparedness for potential epidemics is essential to identify and rectify gaps within current systems. Somalia’s experience offers important lessons for countries experiencing complex humanitarian emergencies that may help prevent and control future cholera outbreaks.
Keywords: cholera, disease outbreaks, emergencies, Somalia.
Citation: Lubogo M; Evans B; Abdinasir A; Sherein E; Muhammad T; Mohamed AM; Hussein A, et al. Response to cholera outbreaks, Somalia, 2017–2019: challenges, interventions and lessons learnt. East Mediterr Health J. https://doi.org/10.26719/emhj.23.078 Received: 24/05/2022; accepted: 05/01/2023
Copyright © World Health Organization (WHO) 2023. Some rights reserved. This work is available under the CC BY-NC-SA 3.0 IGO license https://creativecommons.org/licenses/by-nc-sa/3.0/igo
Introduction
Cholera is a disease of inequity, affecting the world’s most vulnerable and marginalized communities. It is an epidemic-prone disease of global importance (1). The global burden of cholera is unknown because of underreporting (2), but an estimated 3–5 million cholera cases every year cause 100 000–120 000 deaths (3). In the Eastern Mediterranean Region of the World Health Organization (WHO), cholera remains a public health concern, especially in countries facing complex emergencies. In the past decade, 14 out of the 22 countries in the Eastern Mediterranean Region have reported cholera cases; in Afghanistan, Djibouti, Iraq, Pakistan, Somalia, Sudan and Yemen, the numbers have reached epidemic proportions. The regional burden of cholera is also difficult to capture because of weak surveillance systems and underreporting of cases (4). Nevertheless, the number of cases is estimated to be around 188 000 a year (3). In Somalia and other counties in the Horn of Africa, cholera outbreaks are spreading at an alarming rate and affecting communities already suffering from conflicts and droughts (5). Controlling cholera is crucial to achieve the Sustainable Development Goals which call for the good health and well-being of all and a reduction in inequity (6).
Cholera is a multifactorial disease occurring and re-emerging frequently as a result of interaction between different risk factors (7). Identified risk factors fall into four main categories: (i) factors related to water and sanitation such as lack of rainfall and decreased vegetation cover (8), flooding leading to contamination of water sources (9), lack of adequate clean water and proper sanitation facilities because of inadequate infrastructure (10), and poor sanitary practices by communities at risk of cholera infections (11); (ii) sociodemographic factors such as poverty, overcrowding and living in a camp for refugees or internally displaced persons (12); (iii) behavioural factors such as open defecation and funeral practices such as washing the bodies of those who have died from cholera (13); and (iv) gaps in knowledge and false beliefs about cholera infection and transmission, oral cholera vaccines, and cholera case management (14).
In Somalia, the protracted conflict, drought and flooding have resulted in over 2.6 million people living in camps for internally displaced persons where access to safe water and proper sanitation is limited (15). Cholera is endemic in the country and outbreaks occur during both the drought and rainy seasons.
Most of the country, particularly the regions of Shabelle and Juba, is prone to flooding about twice a year leading to contamination of water sources. Moreover, in 2017, Somalia experienced severe drought that affected over 60% of the country and led to severe water and food shortages (16). It was estimated that more than 3 million people were at risk of starvation and malnutrition (17). The limited access to safe water and poor sanitation conditions contributed to Somalia’s worst cholera outbreak in a decade (2,18–20). In January 2017, the Federal Ministry of Health confirmed a cholera outbreak in the Hiran region following the isolation of Vibrio cholera, serotype Ogawa from stool samples of suspected cases. The epidemic spread rapidly to most districts and regions of Somalia and reached its peak during May and June 2017 (19).
This paper aimed to describe the main challenges faced by Somalia during the 2017–2019 cholera outbreaks, highlight Somalia’s response to those challenges with the support of WHO and other partners, and identify lessons learnt. The paper also provides guidelines on how to better prepare for future outbreaks in Somalia and other countries with complex humanitarian emergencies and poor operating environments.
Methods
We undertook a literature review specific to Somalia of available information on the cholera outbreaks of 2017–2019 with particular reference to: preparedness and response; focus group discussions and key informant interviews; and interpretation of the cholera-related indicators in the 3 years of the outbreaks.
Literature review and cholera indicators
The qualitative and quantitative review was undertaken to analyse decision-making, policy, and actions taken during the 2017–2019 cholera emergency in Somalia. The literature search included cholera preparedness and response plans, surveillance records, monitoring and evaluations reports, needs assessments reports, meeting notes, presentations, internal reports, peer-reviewed articles, and relevant grey literature. The data on key indicators including cases, deaths and case fatality rates (CFRs) were collected from the surveillance records of Somalia’s Early Warning and Response Network Surveillance System (EWARN) (21), WHO’s Global Health Observatory and published records (2,18), and cholera situation reports by the WHO Regional Office for the Eastern Mediterranean (19,22). The data were summarized and changes in the indicators over time are presented. A cholera case was defined as a suspected case with V. cholerae 01 and O139 confirmed by stool culture. The cholera CFR was defined as the proportion of cholera-related deaths among total cholera cases during 2017–2019 and was expressed as a percentage.
Focus group discussions and key informant interviews
Participants for 10 key informant interviews and five focus group discussions were selected purposively based on their prominent roles in the health services, and/or their acknowledged understanding and custodianship of the health care system. The key informant interviews and focus group discussions helped provide in-depth information/perspectives for the qualitative review.
Nineteen participants from eight regions in Somalia were included in the focus group discussions, which were held in Mogadishu in 2017 by a trained interviewer who guided the discussion based on pre-identified themes. The interviewer encouraged participants to express their thoughts and ideas freely without interruptions. Pre-identified themes for the focus group discussions included: outbreak detection/confirmation; organization of response; use of reactive oral cholera vaccines; information management; case management; mortality reduction; hygiene measures at the health facility level; involvement of the community to reduce the effect of the disease; surveillance; funeral practices; and three themes related to control of the environment – safe water, safe food and sanitation.
The key informant interviews were done in 2019 mainly with senior staff at the Federal Ministry of Health and with department heads at relevant international agencies who had extensive knowledge of their organization’s involvement in the cholera response.
Both the focus group discussions and key informant interviews were audio-recorded and transcribed separately by two researchers. The transcribed data were analysed thematically by a researcher who was blind to the aims of the focus group discussions and key informant interviews.
Results
Cholera outbreaks in Somalia
In East Africa, particularly the Horn of Africa, and the Middle East, large cholera outbreaks with high mortality are frequently reported (18). The historic trend of cholera in Somalia has not been much studied (23). Although Somalia has long faced cholera outbreaks, the earliest record of a cholera outbreak appears in the WHO’s Global Health Observatory for 1970 (18). For the past 3 decades, almost all small-to-large cholera outbreaks in Somalia coincided with outbreaks globally and in the Eastern Mediterranean Region. However, except for the small-scale outbreaks in 2008–2010 and 2014–2015, the cholera cases, deaths and CFRs in Somalia were higher than the corresponding global and regional averages.
On average in the decade 2010–2019, 22 505 cholera cases and 379 deaths (CFR 1.68%) occurred in Somalia, which is higher than the regional average of 16 918 cases and 133 deaths (CFR 0.79%) and the global average of 9765 cases and 95 deaths (CFR 0.97%). The higher values of the indicators can be attributed to two large cholera epidemics of almost similar scale in Somalia, in 2011 and 2017. Except for in 2002, the cholera CFR in Somalia has always been higher than the global and regional CFR averages (2,18,19,22).
The 2017 cholera outbreak was the largest since 1970, with 78 701 cases and 1163 deaths, mainly among children younger than 5 years. The outbreak was more widespread and severe, encompassing 85 districts in 20 regions within the country, nine of which were classified as partially accessible (urban areas were accessible but not villages) because of political conflict (2,19,22). The highest incidence was in Bay region with 14 964 reported cases, while the lowest was in Sahil with only three cases reported (Figure 1 and Table 1). The overall CFR in 2017 was 1.48%. Of the 78 701 cases and 1163 deaths, 42 987 (56.42%) cases and 582 (51.86%) deaths were reported from the partially accessible regions (Table 1).
The highest peak of the 2017 outbreak was in epidemiological week 22 (29 May–4 June) when more than 5000 cases of cholera were reported. Thereafter, the number of reported cases declined gradually and reached its minimum of 144 cases in epidemiological week 47 (20 November–26 November). After that time, sporadic cases of cholera were reported during December 2017 (22) (Figure 2). This 2017 peak in cases was attributed to a series of unfavourable events that began with heavy rains which caused flash floods that led to contamination of water sources and displacement of communities to camps where access to safe water and proper sanitation was limited. After the flash floods, drought occurred in parts of Somalia, which led to food insecurity and malnutrition among children and resulted in lowered immunity to waterborne infections. Communities did not have enough time to fully recover from each of these hazards, and this situation, together with the weak health system, contributed to the repeated cholera outbreaks with varying degrees of severity.
Cholera cases continued to be reported in 2018 and 2019 (19,22), but the total number of cases was much lower than in 2017 (Table 1 and Figure 2). In 2018, the cumulative total of cholera cases was 6448 with 45 associated deaths (CFR 0.70%) in 23 districts from accessible regions (Banadir and Lower Shabelle) and partially accessible regions (Hiran, Lower Juba, Lower Shabelle, Middle Shabelle and Lower Shabelle) (19,22). In 2019, the cumulative total of cholera cases was 3089 with four associated deaths (CFR 0.13%) from 19 districts in Banadir region and the partially accessible regions of Gedo and Lower Juba (19,22). Overall, the ongoing cholera outbreak that started in December 2017 up to December 2019 has resulted in 13 818 cases and 72 deaths (CFR 0.52%), reported from three states of Somalia (Hirshabelle, Jubaland and South West) and Banadir region (19,24).
Discussion
According to WHO, preparedness, response and post-endemic activities collectively comprise three phases of effective cholera control (25). However, the level of preparedness is the most crucial phase of cholera control and essentially determines the success of an outbreak response (26). Therefore, we identified lessons learnt from the 2017–2019 cholera response in Somalia to provide recommendations on how to better prepare for and respond to future potential outbreaks in the country and other countries with poor operating environments during humanitarian emergencies.
A combination of factors led to Somalia’s severe 2017 cholera outbreak. Protracted conflict contributed to the weakening of Somalia’s health system. Conflict led to displacement of people to camps where access to safe water and sanitation is limited. Severe drought in 2016 and 2017 led to water shortages, displacement, food shortages and malnutrition in children younger than 5 years which in turn led to low immunity.
Our analysis of the trends in the number of cholera cases and deaths and the CFR shows that, despite many years of public health interventions, cholera is still a recurring and important risk to vulnerable communities in Somalia. In 2017, the country experienced the worst cholera outbreak in 5 years, with 78 701 cases and 1163 deaths, mostly in children younger than 5 years (18,19,22).
The Global Task Force for Cholera Control was established in 1991 and revitalized in 2011 as a result of the World Health Assembly resolution WHA64.15, which requested the WHO Director-General to strengthen WHO’s work in this area (27,28). Later in 2017, this task force lunched “Ending cholera: a global roadmap to 2030” and formulated a framework to achieve that target (6). In October 2017, a call to action to fight cholera through implementation of the global roadmap was made by 35 task force partners (27). Despite global efforts to end cholera in Somalia, cholera outbreaks are still reported since conflicts are still ongoing. Therefore, reassessment of cholera preparedness and response plans is important to achieve the goal to end cholera by 2030.
Several countries with complex emergencies have experienced repeated cholera outbreaks and successfully implemented response activities and interventions with the support of WHO and other partners. However these efforts faced challenges and obstacles (29–31). Although Somalia faces a similar situation (19), the humanitarian crisis in Somalia is characterized by multiple hazards occurring in quick succession without any time for full recovery from preceding hazards. Recognizing these challenges and exploring Somalia’s experiences is important to identify and bridge gaps within the current surveillance and response systems at both national and subnational levels. EWARN was launched in Somalia in 2010 but collapsed during ongoing conflict. Until 2017, no reliable surveillance system existed for timely detection of alerts of cholera and other epidemic-prone diseases. With the support of WHO, EWARN was re-activated in 2017 to provide timely detection and response alerts for cholera (32).
We identified several important challenges including: a weak health system; fragile water, sanitation and hygiene (WASH) infrastructure; difficulty in obtaining real-time information; poor resources; and limited funding. However, the most important challenge was conflict, which was responsible for all the other challenges. With support of WHO and other partners, Somalia was able to overcome these challenges and successfully responded to the cholera outbreaks in 2017–2019. However, these outbreaks will not be the last; therefore, ongoing support is vital for the prevention and early detection of, rapid response to and containment of future outbreaks.
Successful interventions that were implemented and contributed to the effective management of the cholera outbreak included: efficient leadership and coordination of epidemic preparedness and response plans at all levels; timely detection and response to alerts; timely dissemination of epidemiological information that was useful for public health action; comprehensive risk assessment; proper case management; enhancement of surveillance and laboratory capacities; strengthening of WASH preparedness; campaigns for community engagement, risk communication; and implementation of campaigns for preventive oral cholera vaccination. The impact of these interventions was evident by the reduction in the total number of cholera cases reported from 78 701 cases in 2017 to 6448 cases in 2018 and 3089 cases in 2019. Similarly, the CFR declined from 1.48% in 2017 to 0.70% in 2018 and 0.13% in 2019.
Based on the forecasting exercise conducted in Somalia in 2018, the total number of reported cases in 2018 was 37.08% less than the best case scenario in which 17 389 individuals were suspected to have cholera the same year. Similar successful experiences were reported from other countries such as Haiti which succeeded in controlling the cholera outbreak following the 2010 earthquake by prioritizing investment in safe water supplies and improved sanitation (33).
Recommendations
Based on our assessment of the experience of responding to the cholera outbreaks in Somalia, the following recommendations are proposed for the country and other countries with similar contexts.
1. Strengthen coordination and leadership to review and update preparedness and response plans for cholera.
2. Integrate diseases surveillance and response systems that include an early warning alert and response network to support timely detection of and response to any alerts of cholera and other epidemic-prone diseases.
3. Increase the number of people with access to safe water and proper sanitation through the establishment of sustainable water systems.
4. Raise awareness of communities in high-risk areas for cholera of the importance of adopting hygienic behaviour; and
5. Perform continuous risk assessments to identify hotspots for cholera and have plans to implement preventive cholera vaccination campaigns.
Acknowledgements
The authors thank the Federal Ministry of Health the Somalia for providing the cholera-related data and permission to submit this manuscript for publication in a peer reviewed journal.
Funding: None.
Competing interests: None declared.
References
1. Cowman G, Otipo S, Njeru I, Achia T, Thirumurthy H, Bartram J, et al. Factors associated with cholera in Kenya, 2008–2013. Pan Afr Med J. 2017;28:101. https://doi.org/10.11604/pamj.2017.28.101.12806
2. Cholera, 2017. Wkly Epidemiol Rec. 2018 93(38):489–500.
3. Risk of cholera in the Eastern Mediterranean Region. Cairo: World Health Organization Regional Office for the Eastern Mediterranean; 2019 (http://www.emro.who.int/health-topics/cholera/index.html, accessed 4 January 2022).
4. Ganesan D, Gupta SS, Legros D. Cholera surveillance and estimation of burden of cholera. Vaccine. 2020; 38 Suppl 1:A13–A17. https://doi.org/10.1016/j.vaccine.2019.07.03
5. Green A. Cholera outbreak in the Horn of Africa. Lancet. 2017;389(10085):2179. https://doi.org/10.1016/S0140-6736(17)31541-6
6. Ending cholera: a global roadmap to 2030 control. Geneva: Global Task Force on Cholera Control; 2017 (https://www.who.int/cholera/publications/global-roadmap/en/, accessed 4 December 2019).
7. Ripoll S. Contextual factors shaping cholera transmission and treatment-seeking in Somalia and the Somali region of Ethiopia. Brighton: Social Science in Humanitarian Action; 2017.
8. Rebaudet S, Sudre B, Faucher B, Piarroux R. Environmental determinants of cholera outbreaks in inland Africa: a systematic review of main transmission foci and propagation routes. J Infect Dis. 2013;208 Suppl 1:S46–54. https://doi.org/10.1093/infdis/jit195
9. Rieckmann A, Tamason CC, Gurley ES, Rod NH, Jensen PKM. Exploring droughts and floods and their association with cholera outbreaks in sub-Saharan Africa: a register-based ecological study from 1990 to 2010. Am J Trop Med Hyg. 2018;98(5):1269–74. https://doi.org/10.4269/ajtmh.17-0778
10. Mengel MA, Delrieu I, Heyerdahl L, Gessner BD. Cholera outbreaks in Africa. Curr Top Microbiol Immunol. 2014;379:117–44. https://doi.org/10.1007/82_2014_369
11. Kwesiga B, Pande G, Ario AR, Tumwesigye NM, Matovu JKB, Zhu BP. A prolonged, community-wide cholera outbreak associated with drinking water contaminated by sewage in Kasese District, western Uganda. BMC Public Health. 2017;18(1):30. https://doi.org/10.1186/s12889-017-4589-9.
12. Swaddiwudhipong W, Ngamsaithong C, Peanumlom P, Hannarong S. An outbreak of cholera among migrants living in a Thai-Myanmar border area. J Med Assoc Thai. 2008;91(9):1433–40.
13. Ringane A, Milovanovic M, Maphakula D, Makete F, Omar T, Martinson N, et al. An observational study of safe and risky practices in funeral homes in South Africa. S Afr Med J. 2019;109(8):587–91. https://doi.org/10.7196/SAMJ.2019.v109i8.13523
14. Taylor DL, Kahawita TM, Cairncross S, Ensink JH. The impact of water, sanitation and hygiene interventions to control cholera: a systematic review. PLoS One. 2015;10(8):e0135676. https://doi.org/10.1371/journal.pone.0135676
15. Camp Coordination and Camp Management (CCCM) Cluster. Somalia dashboard. Internally displaced persons (IDP). Geneva: United Nations High Commissioner for Refugees; 2018 (https://data2.unhcr.org/en/documents/download/62286, accessed 4 January 2022).
16. Somalia drought impact and needs assessment. Volume 1. Synthesis report. New York: United Nations Development Programme; 2017 (https://www.undp.org/publications/somalia-drought-impact-and-needs-assessment, accessed 4 January 2022).
17. Horn of Africa: humanitarian impacts of drought–issue 6. New York: United Nations Office for the Coordination of Humanitarian Affairs; 2017 (https://reliefweb.int/sites/reliefweb.int/files/resources/HOA_drought_update_16June2017.pdf accessed 4 January 2022).
18. Global Health Observatory data repository: explore a world of health data. Geneva: World Health Organization; 2022 (https://www.who.int/data/gho, accessed 4 January 2022).
19. Cholera situation in Somalia. Cairo: World Health Organization Regional Office for the Eastern Mediterranean; 2020 (http://www.emro.who.int/pandemic-epidemic-diseases/outbreaks/outbreaks-archive.html, accessed 4 January 2022).
20. Legros D. Global cholera epidemiology: opportunities to reduce the burden of cholera by 2030. J Infect Dis. 2018;218(Suppl_3):S137–40. https://doi.org/10.1093/infdis/jiy486
21. Somalia – EWARN May 2020: COVID-19 information note 2. Cairo: World Health Organization Regional Office for the Eastern Mediterranean; 2020 (https://reliefweb.int/report/somalia/somalia-ewarn-may-2020-covid-19-information-note-2, accessed 4 January 2022).
22. Cholera situation in Somalia, December 2017. Cairo: World Health Organization Regional Office for the Eastern Mediterranean; 2017 (https://reliefweb.int/sites/reliefweb.int/files/resources/cholera_situation_update_somalia_december_2017.pdf, accessed 4 January 2022).
23. Somalia integrated cholera response plan for central-south zones. April to June 2017. New York: United Nations Children’s Fund; 2017 (https://reliefweb.int/report/somalia/unicef-somalia-integrated-cholera-response-plan-central-south-zones-april-june-2017, accessed 4 January 2022).
24. Weekly AWD/cholera situation report – Somalia. Epidemiological week 22 (21–31 May 2020). Cairo: World Health Organization Regional Office for the Eastern Mediterranean; 2020 (https://reliefweb.int/report/somalia/weekly-awdcholera-situation-report-somalia-epidemiological-week-22-21-31-may-2020, accessed 4 January 2022).
25. Prevention and control of cholera outbreaks: WHO policy and recommendations. Geneva: World Health Organization; 2010.
26. Ateudjieu J, Yakum MN, Goura AP, Nafack SS, Chebe AN, Azakoh JN, et al. Health facility preparedness for cholera outbreak response in four cholera-prone districts in Cameroon: a cross sectional study. BMC Health Serv Res. 2019;19(1):458. https://doi.org/10.1186/s12913-019-4315-7
27. The Global Task Force on Cholera Control. Cholera unveiled. Geneva: World Health Organization; 2020 (http://whqlibdoc.who.int/hq/2003/WHO_CDS_CPE_ZFK_2003.3.pdf, accessed 4 January 2022).
28. Report of first meeting of the global task force for cholera control, 26–27 June 2014, Chavannes-de-Bogis, Switzerland. Geneva: World Health Organization; 2014 (https://apps.who.int/iris/handle/10665/205122, accessed 4 January 2022).
29. Ayenigbara IO, Ayenigbara GO, Adeleke RO. Contemporary Nigerian public health problem: prevention and surveillance are key to combating cholera. GMS Hyg Infect Control. 2019;14:Doc16. https://doi.org/10.3205/dgkh000331
30. Lucien MAB, Adrien P, Hadid H, Hsia T, Canarie MF, Kaljee LM, et al. Cholera outbreak in Haiti: epidemiology, control, and prevention. Infect Dis Clin Pract. 2019;27(1):3–11.
31. Ngwa MC, Liang S, Mbam LM, Mouhaman A, Teboh A, Brekmo K, et al. Cholera public health surveillance in the Republic of Cameroon – opportunities and challenges. Pan Afr Med J. 2016;24:222. https://doi.org/10.11604/pamj.2016.24.222.8045
32. Health workers trained on electronic disease early warning surveillance systems. Cairo: World Health Organization Regional Office for the Eastern Mediterranean: 2018 (/pandemic-epidemic-diseases/news/health-workers-trained-on-electronic-disease-early-warning-surveillance-systems.html, accessed 4 January 2022).
33. Robbins A. Lessons from cholera in Haiti. J Public Health Policy. 2014;35(2):135–6. https://doi.org/10.1057/jphp.2014.5
Table 1. Cases of and deaths from cholera and CFR, by region, during cholera outbreaks, Somalia, 2017–2019
Figure 1. Regions most affected by cholera, Somalia, 2017
Figure 2. Trend in cholera cases and case fatality rate during cholera outbreaks, Somalia, 2017–2019
Knowledge and views of doctors and veterinarians about One Health, Türkiye
Zeynep Ö. Özgüler1 and Dilek Aslan2
1General Directorate of Public Health, Ministry of Health of Türkiye, Ankara, Türkiye. 2Department of Public Health, Hacettepe University Faculty of Medicine, Ankara, Türkiye. (Correspondent to Zeynep Ö. Özgüler:
Abstract
Background: One Health is a multisectoral and interdisciplinary concept that acknowledges interconnectedness between people, animals and their shared environments. Understanding the views of doctors and veterinarians may help promote One Health principles.Aim: To assess the knowledge and views of doctors and veterinarians about One Health in Türkiye.
Methods: This was a descriptive mixed-methods study. Quantitative data were collected through an email questionnaire sent to members of the Ankara Chambers of Medicine and Veterinary Medicines (professional bodies). Qualitative data were obtained through focus group discussions with boards of directors of both these chambers. Recordings were transcribed and the data were categorized in line with the questionnaire.
Results: In total, 74 doctors and 221 veterinarians responded to the questionnaire. Few of veterinarians had not heard of One Health (6.3%) while most doctors had not heard of it (63.5%), P < 0.001. While most of the doctors and veterinarians had not received training on One Health (95.9% and 71.5%, respectively), significantly more veterinarians had received training (P < 0.001). Most of the veterinarians and a few doctors had applied One Health in their work (73.3% and 35.1%, respectively; P < 0.001). Although participants mentioned other disciplines related to One Health, they had not been involved in any cooperative or collaborative work with them.
Conclusion: The application of one-health principles by the doctors and veterinarians was limited, and few collaborated with other disciplines. Joint training and further professional and educational integration are needed to support the implementation of One Health.
Keywords: One Health, doctors, veterinarians, Türkiye.
Citation: Özgüler ZO; Aslan D. Knowledge and views of doctors and veterinarians about One Health: Türkiye. East Mediterr Health J. 2023;29(x):xxx–xxx. https://doi.org/10.26719/emhj.23.XXX Received: 22/07/22; accepted: 04/01/23
Copyright © World Health Organization (WHO) 2023. Some rights reserved. This work is available under the CC BY-NC-SA 3.0 IGO license https://creativecommons.org/licenses/by-nc-sa/3.0/igo
Introduction
One Health is a multisectoral and interdisciplinary approach – at the regional, national and global level – to achieve the best health outcomes, recognizing the interconnectedness of human, animal, plant life and their shared environments. Today, the definition of the One Health has been expanded to cover topics such as food safety, poverty, gender equality, health system strengthening, infectious diseases, chronic diseases, toxicology, ecology, agriculture, sustainability, preventive medicine, economics, anthropology and social sciences. It is expected that professionals working in the above-mentioned fields will work in cooperation (1–3). The coronavirus disease 2019 (COVID-19) pandemic emphasized the importance of prioritization of transdisciplinary systems, which can be attained through one-health principles (4–6). Although the one-health approach is compatible with the holistic definition of health, One Health is not fully understood and implemented in health sciences (7–9). Moreover, further studies are needed on One Health to help inform related policies and practices (10). Doctors and veterinarians have important roles to play in monitoring, planning, detecting, teaching, sharing information and policy-making related to diseases and response to disease threats, which makes it essential for them to have knowledge and awareness of One Health to improve health-related policy, research and the practical implementation of One Health. One Health can facilitate collaborations between these health professionals and other related fields (11,12).
Understanding the views of doctors and veterinarians on One Health is a good starting point to promote and implement one-health principles to combat health threats. We hypothesized that medical doctors and veterinarians in Ankara, Türkiye might lack sufficient awareness and knowledge of the concept of One Health. Therefore, the aim of this study was to assess the knowledge, awareness and views of doctors and veterinarians in Ankara, Türkiye about One Health and its application.
Methods
In this descriptive study, quantitative and qualitative data were collected. For the quantitative component, after reviewing the relevant literature, documents and reports, we developed a questionnaire to elicit the knowledge and views of doctors and veterinarians about One Health and their use of it. The questionnaire was sent via email to members of the Ankara Chambers of Medicine and Veterinary Medicine between 24 February and 24 April 2020. In total, 1069 members viewed the questionnaire. All who completed the process by agreeing to fill out the questionnaire were included in the study.
Members of the boards of directors of the Ankara Chambers of Medicine and Veterinary Medicine were also interviewed online to explore the ideas and recommendations in more detail. The participants were asked about: (i) the scope of One Health; (ii) the awareness of One Health in Türkiye; (iii) ways to increase awareness of One Health; (iv) the responsibility of the professional chambers (bodies) to develop the concept of One Health; and (v) the activities related to One Health that could be undertaken in their own professional chamber. The online meeting with six board members of the Ankara Chamber of Medicines was held on 13 June 2020. The meeting with three board members of the Ankara Chamber of Veterinary Medicine was held on 22 June 2020. With participants’ permission, video recordings were done and transcribed. Recordings were re-watched and transcripts were re-read. Preliminary insights and further discussion notes were summarized following the topics of the questionnaire and categorized into different themes. The insights that emerged were grouped under subcategories and themes.
SPSS version 23.0 was used to evaluate the data. The chi-squared and Fisher exact tests were used to compare the differences between doctors and veterinarians in their knowledge and responses. P < 0.05 was considered statistically significant.
Approval for this study was obtained from the Non-interventional Clinical Research Ethics Committee of Hacettepe University (decision number: 2019/22-16).
Results
A total of 295 people completed the questionnaire: 74 (25.1%) doctors and 221 (74.9%) veterinarians. Of the doctors, 29 (39.2%) worked at universities, 21 (28.4%) at the Ministry of Health and 17 (23.0%) in private health institutions. Of the veterinarians, 84 (38.0%) worked at the Ministry of Agriculture and Forestry, 71 (32.1%) at universities and 41 (18.6%) were self-employed.
Most of the doctors (63.5%) but only a few of the veterinarians (6.3%) had never heard of One Health; P < 0.001 (Table 1). Few doctors or veterinarians had received training in One Health, although significantly more veterinarians had received training than doctors (25.3% versus 4.1%; P < 0.001). Of the veterinarians who had received training, most received it at a congress, symposium or conference, or during their undergraduate education (Table 1).
When asked which areas One Health could contribute to, most participants mentioned: control of zoonoses (96.9%); facilitation of information-sharing about common health problems in humans and animals (95.9%); and ensuring food hygiene and inspection (94.2%). Other areas mentioned were: aiding detection and prevention of environmental pollution; combating antibiotic resistance; providing healthy/standardized laboratory animals; education; helping maintain ecological balance; protection against electromagnetic pollution; protecting social and mental health, animal health and nutrition; promoting a hygiene culture; supporting occupational safety; controlling pandemics, pesticides, carcinogens and obesogens; and supporting biomedical research and ecotoxicology (Table 1).
With regard to consideration of One Health during their work, 35.1% (26/74) of doctors and 73.3% (162/221) of veterinarians said that they applied the concept, whether always, often or rarely. When asked how One Health was applied in their work, 58.3% of the doctors and 55.3% of the veterinarians stated that this was through information-sharing and training activities. Other aspects of their work mentioned most were food safety and food consumption habits, and diagnosis and treatment practices (Table 1).
Participants were asked to name the three zoonotic diseases they thought were most common in Türkiye. The zoonotic disease listed by doctors were: brucellosis (56.8%), anthrax (32.4%), salmonellosis (29.7%), hydatid cyst (29.7%), rabies (28.4%), and tick-borne diseases–Crimean–Congo haemorrhagic fever, Lyme disease – (24.3%). The diseases cited by veterinarians were: brucellosis (88.7%), salmonellosis (43.9%), toxoplasmosis (35.3%), rabies (28.9%), anthrax (28.1%) and hydatid cyst (15.8%).
Of the doctors, 20.3% worked in zoonotic diseases, 60% of whom said that the most common zoonotic diseases they had encountered in their work were hydatid cyst, brucellosis and anthrax. In comparison, 50.7% of the veterinarians worked in zoonotic diseases and the zoonotic disease they encountered mostly were brucellosis, tuberculosis, and rabies. Most veterinarians (62.5%) said that they had cooperated with different sectors or professions during their work on zoonotic diseases, mostly with other veterinarians. Of the doctors who had worked in zoonotic diseases, 40.0% said that they had cooperated with different sectors or professions during their work.
When asked about the disciplines related to One Health, medicine and veterinary medicine were the most commonly mentioned disciplines by both doctor and veterinarians (Table 1). Other disciplines they considered were related to One Health were public health, ecology, epidemiology, biology, social sciences, anthropology, bioinformatics, bioengineering, environmental sciences, economics, political sciences, zoology, dentistry, pharmacy, agricultural sciences, food engineering, food hygiene, statistics ergonomics, philosophy, genetics and chemistry.
With regard to factors that could contribute to the development of One Health, joint training activities for faculties related to medicine, veterinary medicine, public health, and environmental health were mentioned by most of the participants, as was collaborative research for the development and evaluation of new diagnostic methods, drugs and vaccines for disease prevention and control across species (Table 1).
Most doctors and veterinarians (90.5%) thought that the development of One Health was necessary for the country as a means of health protection and promotion. Other reasons given were developing new diagnoses and treatments and coordinating interdisciplinary and interprofessional studies.
Participants were asked their opinion on the best approach to developing the concept of One Health in undergraduate and graduate training. More doctors (50.4%) than veterinarians (25.8%) thought that One Health should be included in a general framework integrated into health education at the undergraduate level. More veterinarians than doctors thought that there should be subject-specific teaching of specialties, including One Health, but with interdisciplinary communication and cooperation. For graduate levels, most veterinarians and doctors supported subject-specific teaching of specialties (Table 2).
Participants were asked about the role of their professional chambers in relation to One Health: 56 doctors (75.7%) thought that the Chamber of Medicine should have some responsibility for the implementation of One Health, while 11 (14.9%) said that their chamber already undertook action on One Health. Of the veterinarians, 172 (77.8%) thought that the Ankara Chamber of Veterinary Medicine should have some responsibility for implementing One Health, while 75 (33.9%) said that their chamber had already undertaken action on this.
The opinions of members of the boards of directors of the professional chambers for medicine and veterinary medicine about One Health are given in Box 1. Responses to the question on the scope of One Health focused on the joint evaluation of the concept from different disciplines with emphasis on multidisciplinary and multisector involvement (Box 1). The participants considered that awareness of the concept of One Health in Türkiye was low and that there was a need to simultaneously raise awareness in both health professionals and the public. In order to increase awareness of the concept of One Health, the focus group participants suggested the use of social media and implementation of promotion and training actions. With regard to the responsibilities of the professional chambers to support the development of the concept of One Health, most participants emphasized actions that require the chambers to act jointly, not alone (Box 1).
Discussion
This study gathered quantitative and qualitative information on use of, awareness of, views on and approaches to One Health among doctors and veterinarians working in different fields and areas in Türkiye.
More than half of the pathogens that cause disease in humans are animal-borne, which emphasizes the impact of zoonotic infectious diseases on human health (13–16). Hence, most of the doctors and veterinarians who applied the concept of One Health in their work said that they applied it with zoonotic diseases, although more than half of both groups did this through information sharing and training activities. No doctors and only a few veterinarians applied One Health through interprofessional or interinstitutional coordination. The study indicated that the doctors and veterinarians did not have many joint communication or common working environments at policy-making institutions. The board members of both professional chambers considered that the following activities could be carried out to develop One Health further: joint training of faculties of medicine, veterinary medicine, and schools of public health and environmental health; joint research; and establishment of communication platforms for related professions and intersectoral collaborations.
The knowledge of our respondents of the most common zoonotic diseases in Türkiye concurred with the study of the Turkish Institute for Health Policies (17). Neither group collaborated much with different professions or sectors apart from other veterinarians and doctors. Other disciplines should also be included while planning research and developing policy related to One Health (14,18). Systematic planning to provide continuous communication between different disciplines and sectors can contribute to collaborative networks and research. Intersectoral application of the one-health concept provides successful results against a common health threat when good intersectoral communication and trust are ensured (19). Our participants considered that integrated surveillance systems and joint training activities could contribute to the development of One Health. Participants believed that One Health was necessary for health protection and promotion. After the avian influenza pandemic, the importance of communication and coordination between sectors and institutions was better understood, and local and international conferences and agreements focused on the pandemics (14). In addition, integrated systems with a one-health approach started to be established to early detect, and effectively prevent and control emerging, re-emerging or increasing infectious diseases that can cause epidemics and pandemics, such as severe acute respiratory syndrome, avian influenza and dengue. Furthermore, it has become essential to make these systems sustainable by expanding them both at local and international levels (14,20), and sustainable workflows are needed for the joint processing of data and unified implementation of actions in light of the information created (14,21).
The one-health concept is not only limited to the relations between animal and human health, but also to the environment. Examining the effects of environmental factors on disease dynamics and effective use of surveillance programmes that include animal and the environmental data can increase our understanding of related diseases and help develop timely interventions (13,22,23). Our participants thought that the environment was neglected in their work. Intersectoral and interprofessional communication and joint health data monitoring and analysis are needed to create unified outputs, understand the relationship between human–animal–environment health, evaluate disease transmission and create an adequate response (1,2,14).
Although there was a consensus on One Health gaining importance in the fight against antibiotic misuse, most participants have not applied it. Trans-disciplinary and trans-sectoral strategies to reduce antibiotic use in animals and humans under a one-health approach can support plans and policies on antimicrobial resistance (22–25).
More than half of the doctors who participated in the study had never heard of One Health and had never thought of applying the concept in their practices. Furthermore, the professionals who applied it had only implemented the concept through information-sharing and educational activities.
For undergraduate education, general training was preferred, while for postgraduate education, specialized training and subject-specific education was preferred. Including the one-health approach in medicine and veterinary curricula will likely have positive results and lead to greater professional practice (14). Postgraduate public health education can help professionals prevent future epidemics by establishing programmes that evaluate human–animal–environmental health issues in connection with a multidisciplinary education (26).
Both professional chambers had declared that One Health training, legislation and practice should be carried out. However, most participants of both groups agreed that their professional chambers had not undertaken action to support the implementation of One Health. Planning joint action with related professional chambers, implementing the planed steps in a sustainable way and better dissemination of information might help both chambers.
Our study has some limitations. First, the descriptive nature of the study limits the generalizability of our results. Second, we used an online survey which limited our access to the professionals and in-depth examination of the participants’ views. Further studies are needed to include other related professionals such as those in environmental health, food-related sciences and pharmaceutical sciences. Despite these limitations, this study helped us better understand the awareness of veterinarians and doctors of One Health and their views on the concept.
Acknowledgements
We thank all the veterinarians, doctors and their professional boards for participating in the study.
This study is based on the master’s thesis in public health of Zeynep Özge Özgüler at Hacettepe University. Part of the thesis results was communicated as a poster presentation at the 14th European Public Health Conference (virtual), 10–12 November 2021.
Funding: None.
Competing interests: None declared.
References
1. Baum SE, Machalaba C, Daszak P, Salerno RH, Karesh WB. Evaluating One Health: are we demonstrating effectiveness? One Health. 2017;3:5–10. https://doi.org/10.1016/j.onehlt.2016.10.004
2. Binot A, Duboz R, Promburom P, Phimpraphai W, Cappelle J, Lajaunie C, et al. A framework to promote collective action within the One Health community of practice: using participatory modelling to enable interdisciplinary, cross-sectoral and multi-level integration. One Health. 2015;1:44–8. https://doi.org/10.1016/j.onehlt.2015.09.001
3. Katz RL, Fernandez JA, McNabb SJ. Disease surveillance, capacity building and implementation of the International Health Regulations (IHR 2005). BMC Public Health. 20103;10(1):S1. https://doi.org/10.1186/1471-2458-10-S1-S1
4. Ruckert A, Zinszer K, Zarowsky C, Labonté R, Carabin H. What role for One Health in the COVID-19 pandemic? Can J Public Health. 2020;111(5):641–4. https://doi.org/10.17269/s41997-020-00409-z
5. Wu Q, Li Q, Lu J. A One Health strategy for emerging infectious diseases based on the COVID-19 outbreak. J Biosaf Biosecurity. 2022;4(1):5–11. https://doi.org/10.1016/j.jobb.2021.09.003
6. Ferri M, Lloyd-Evans M. The contribution of veterinary public health to the management of the COVID-19 pandemic from a One Health perspective. One Health. 2021;12:100230. https://doi.org/10.1016/j.onehlt.2021.100230
7. Mackenzie JS, McKinnon M, Jeggo M. One Health: from concept to practice. Confronting Emerg Zoonoses. 2014:163–89. https://doi.org/10.1007/978-4-431-55120-1_8
8. Gallagher CA, Keehner JR, Hervé-Claude LP, Stephen C. Health promotion and harm reduction attributes in One Health literature: a scoping review. One Health. 2021;13:100284. https://doi.org/10.1007/978-4-431-55120-1_8
9. Pettan-Brewer C, Martins AF, de Abreu DPB, Brandão APD, Barbosa DS, Figueroa DP, et al. From the approach to the concept: One Health in Latin America: experiences and perspectives in Brazil, Chile, and Colombia. Front Public Health. 2021;9:687110. https://doi.org/10.3389/fpubh.2021.687110
10. Degeling C, Rock M. Qualitative research for One Health: from methodological principles to impactful applications. Front Vet Sci. 2020;7:70. https://doi.org/10.3389/fvets.2020.00070
11. Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. From “one medicine” to “One Health” and systemic approaches to health and well-being. Prev Vet Med. 2011;101(3):148–56. https://doi.org/10.1016/j.prevetmed.2010.07.003
12. Errecaborde KM, Macy KW, Pekol A, Perez S, O’Brien MK, Allen I, et al. Factors that enable effective One Health collaborations: a scoping review of the literature. PLoS One. 2019;14(12):e0224660. https://doi.org/10.1371/journal.pone.0224660
13. Dahal R, Kahn L. Zoonotic diseases and One Health approach. Epidemiol Open Access. 2014;4(2):1. https://doi.org/10.4172/2161-1165.1000e115
14. Jeggo M, Mackenzie JS. Defining the future of One Health. Microbiol Spectr. 2014;2(1):OH-0007-2012. https://doi.org/10.1128/microbiolspec.OH-0007-2012
15. Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–3. https://doi.org/10.1038/nature06536
16. Woolhouse MEJ, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. 2005;11(12):1842–7. https://doi.org/10.3201/eid1112.050997
17. Arı HO, İşlek E, Bilir Uslu MK, Özatkan Y, Karakaş F, Yıldırım HH, et al. The monetary impact of zoonotic diseases on society: the Turkish case. Ankara Univ Vet Fak Derg. 2022;69(1):9–15. https://doi.org/10.33988/auvfd.789598
18. Zinsstag J, Schelling E, Waltner-Toews D, Whittaker M, Tanner M, CAB International, editors. One Health: the theory and practice of integrated health approaches. Wallingford, UK , Boston, USA: CABI; 2015:447. https://doi.org/10.1079/9781780643410.0000
19. Rubin C, Dunham B, Sleeman J. Making One Health a reality – crossing bureaucratic boundaries. Microbiol Spectr. 2014;2(1):OH-0016-2012. https://doi.org/0.1128/microbiolspec.OH-0016-2012
20. Keusch GT, Pappaioanou M, Gonzalez MC, Scott KA, Tsai P, editors. Sustaining global surveillance and response to emerging zoonotic diseases. Washington (DC): National Academies Press (US); 2009 https://doi.org/10.17226/12625
21. Davis MF, Rankin SC, Schurer JM, Cole S, Conti L, Rabinowitz P, et al. Checklist for One Health Epidemiological Reporting of Evidence (COHERE). One Health. 2017;4:14–21. https://doi.org/10.1016/j.onehlt.2017.07.001
22. Mwangi W, de Figueiredo P, Criscitiello MF. One Health: addressing global challenges at the nexus of human, animal, and environmental health. PLoS Pathog. 2016;12(9). https://doi.org/10.1371/journal.ppat.1005731
23. Ryu S, Kim BI, Lim JS, Tan CS, Chun BC. One Health perspectives on emerging public health threats. J Prev Med Pub Health. 2017;50(6):411–4. https://doi.org/10.3961/jpmph.17.097
24. Davies J. Antibiotic resistance in and from nature. Microbiol Spectr. 2013;1(1): OH-0005-2012. https://doi.org/10.1128/microbiolspec.OH-0005-2012
25. Relman DA. Microbial genomics and infectious diseases. N Engl J Med. 2011;365(4):347–57. https://doi.org/10.1056/NEJMra1003071
26. Kahn LH. The need for One Health degree programs. Infect Ecol Epidemiol. 2011;1. https://doi.org/10.3402/iee.v1i0.7919
Table 1. Knowledge and views of doctors and veterinariansabout One Heath, Türkiye
|
Knowledge and views about One Health |
Doctors |
Veterinarians |
P |
|
No. (%) |
No. (%) |
||
|
Knowledge of the concept |
(n = 74) |
(n = 221) |
|
|
Full knowledge |
5 (6.8) |
115 (52.0) |
< 0.001 |
|
Knew the definition |
9 (12.2) |
69 (31.2) |
|
|
Heard of it before |
13 (17.6) |
23 (10.4) |
|
|
Never heard of it |
47 (63.5) |
14 (6.3) |
|
|
Received training on One Healtha |
|||
|
No |
71 (95.9) |
158 (71.5) |
< 0.001 |
|
Yes |
3 (4.1) |
56 (25.3) |
|
|
Place of trainingb |
(n = 3) |
(n = 56) |
|
|
University (undergraduate degree) |
– |
25 (44.6) |
0.3485 |
|
University (graduate degree) |
– |
12 (21.4) |
|
|
Continuing vocational education |
2 (66.7) |
21 (37.5) |
|
|
Congress/symposium/conference |
1 (33.3) |
28 (50.0) |
|
|
Disciplines related to One Healthc,d |
(n = 74) |
(n = 221) |
|
|
Medicine |
71 (95.9) |
210 (95.0) |
0.999 |
|
Veterinary sciences |
72 (97.3) |
215 (97.3) |
0.999 |
|
Public health |
67 (90.5) |
206 (93.2) |
0.449 |
|
Epidemiology |
57 (77.0) |
184 (83.3) |
0.231 |
|
Social sciences |
51 (68.9) |
89 (40.3) |
< 0.001 |
|
Ecology |
65 (87.8) |
162 (73.3) |
0.011 |
|
Biology |
58 (78.4) |
147 (66.5) |
0.055 |
|
Areas in which they applied One Health during their workb,c,d |
(n = 26) |
(n = 162) |
|
|
Zoonotic diseases |
15 (57.7) |
134 (82.7) |
0.003 |
|
Food safety |
4 (15.4) |
34 (21.0) |
0.509 |
|
Antibiotic resistance |
2 (7.7) |
18 (11.1) |
0.599 |
|
How they applied One Health during their workb |
(n = 24) |
(n = 159) |
|
|
Restricting the use of antibiotics and raising awareness |
1 (4.2) |
6 (3.8) |
0.642 |
|
Vaccination studies |
– |
8 (5.0) |
|
|
Information and training activities |
14 (58.3) |
88 (55.3) |
|
|
Scientific research |
1 (4.2) |
3 (1.9) |
|
|
Food safety and food consumption habits |
2 (8.3) |
20 (12.6) |
|
|
Personal hygiene and biosecurity measures |
2 (8.3) |
5 (3.1) |
|
|
Diagnosis and treatment |
4 (16.7) |
20 (12.6) |
|
|
Interinstitutional and interprofessional coordination |
– |
9 (5.7) |
|
|
Contributors to the development of One Healthc |
(n = 74) |
(n = 221) |
|
|
Medicine, veterinary and public and environmental health joint training activities |
69 (93.2) |
217 (98.2) |
0.033 |
|
Collaborative research for the development and evaluation of new |
60 (81.1) |
193 (87.3) |
0.183 |
|
Integrated surveillance systems |
21 (28.4) |
98 (44.3) |
0.015 |
|
Development of collaboration between medical, veterinary |
53 (71.6) |
123 (55.7) |
0.015 |
|
Journals, conferences and communication efforts among human, |
14 (18.9) |
28 (12.7) |
0.183 |
|
Areas One Health can contribute toc,d |
|
|
|
|
Control of zoonoses |
69 (93.2) |
217 (98.2) |
0.094 |
|
Ensuring food hygiene and inspection |
67 (90.5) |
211 (95.5) |
0.115 |
|
Detection and prevention of environmental pollution |
65 (87.8) |
155 (70.1) |
0.002 |
|
Facilitation of information-sharing on common health problems |
71 (95.9) |
212 (95.9) |
0.999 |
|
Provision of healthy, standardized laboratory animals |
37 (50.0) |
121 (54.8) |
0.478 |
|
Combating of antibiotic resistance |
54 (73.0) |
190 (86.0) |
0.011 |
a Seven veterinarians answered that they did not remember.
b Conditional question, participants who answered “yes” to the previous questions answered this question.
c Multiple choice question.
d Space was provided for participants to give their other answers and comments; these answers are reported in the results section of this paper.
Table 2. Views of doctors and veterinarians about undergraduate and graduate degree training for One Health, Türkiye
|
Type of training |
Undergraduate degree education |
Graduate degree education |
||||
|
Doctors |
Veterinarians |
P |
Doctors |
Veterinarians |
P |
|
|
|
No. (%) (n = 74) |
No. (%) (n = 221) |
|
No. (%) (n = 74) |
No. (%) (n = 221) |
|
|
Further, more in-depth differentiated and specialized |
6 (8.1) |
38 (17.2) |
0.001 |
12 (16.2) |
55 (24.9) |
0.091 |
|
Subject-specific teaching of topics and specialties, |
28 (37.8) |
121 (54.8) |
45 (60.8) |
139 (62.9) |
||
|
A general framework with different disciplines integrated |
38 (51.4) |
57 (25.8) |
15 (20.3) |
25 (11.3) |
||
|
Othera |
2 (2.7) |
5 (2.3) |
2 (2.7) |
2 (0.9) |
||
a Space was provided for participants to give their answers and comments; these answers are reported in the results section of this paper.
|
Box 1. Examples of participants’ views of OneHealth in focus group discussions, Türkiye |
|
Question 1: What is the scope of the One Health concept according to you? |
|
Necessity of One Health “…It is necessary to fight with a One Health approach for healthier environment for epidemics and zoonotic diseases. At the very least, that way we can eliminate zoonoses and contribute to both health and economy.” (Veterinarian) “… In a world where animal health does not exist, it is not possible to talk about healthy humans and societies…” (Veterinarian) |
|
Multidisiplinary and multisectoral involvment “A multidisciplinary education and practice should be achieved.” (Veterinarian) “…interaction between all organic and inorganic structures sffects health as a whole... The environment is the neglected element here…” (Doctor) |
|
Question 2: Do you think the concept of One Health is known in the country? |
|
Low professional and public awerness “This concept has only just begun to be developed in Türkiye...” (Veterinarian) “…There is low awareness not only in society but also in people whose work is related to basic sciences.” (Doctor) |
|
Recommendations to raise professional awareness “It would be good to plan a symposium.” (Doctor) “Students want to organize information meetings and symposiums about One Health. The students are trying to do something about this issue.” (Veterinarian) |
|
Question 3: What can be done to increase awareness of the concept of One Health? |
|
Recommendations to raise public awareness “Information meetings can be held and announced on social media. I think that it is possible to advertise public service announcements and organize events that include all veterinary and human medicine professions and other health-related organizations.” (Veterinarian) “I think that the organizations where professional bodies come together, rather than individually, and posting on social media and Twitter using hashtags can make attract more attention. There may be celebrities and public announcements that could be effective in this regard.” (Veterinarian) |
|
Professional training “…It can be included in the faculty curricula.” (Doctor) “… The World Health Organization acts as a catalyst. Zoonoses are increasing and in time it [professional training] will have to be imposed… WHO, unions and universities will be the drivers of this.” (Doctor) “…The process will develop in its own in time, even without having to push it; but there should be One Health training in multidisciplinary studies before that happens” (Doctor) |
|
Questions 4: What responsibilities fall on the professional chambers for the development ofOne Health? Question 5:What activities do you intend to carry out in your professional chamber? |
|
Coming together to take action “… Stakeholders need to do it together and political sanctions are needed. Dialogue and communication between chambers would increase the success rate…It is necessary to work together, thinking that we can do nothing alone.” (Veterinarian) “…There is a solidarity network with other chambers, such as the chamber of dentists, veterinarians and pharmacists. We can act as catalysts with other professional chambers. Health requires a holistic approach. We can only offer suggestions by making connections with nongovernmental organizations and other chambers to raise public awareness, but we are not the ones who will determine the curriculum.” (Doctor) |
|
Recommendations for action “What can be done concretely? …The curriculum should be inclusive. If necessary, suggestions can be made by writing to the World Medical Association through the Turkish Medical Association... It is necessary to act as a natural advisory board. One representative from each chamber can take part in a One Health-related city council...” (Doctor) “I think the use of social media is effective. Contact was made with other health profession associations, but it became stagnant due to COVID-19… Activities can be organized for social media visibility such as social gatherings, trips and cartoon competitions.” (Doctor) |
A pilot situation analysis of mapping research ethics governance in Pakistan
Aamir Jafarey1, Sualeha Shekhani1, Faiz Raza2, Sumera Naz3
Centre of Biomedical Ethics and Culture, Sindh Institute of Urology & Transplantation, Karachi, Pakistan (Correspondence: A.M. Jafarey:
Abstract
Background: Mapping of ethical governance structures is useful in identifying strengths and weaknesses in order to uphold integrity and ensure standardization. However, reliable countrywide data about ethical review committees (ERCs) is unavailable in Pakistan.
Aims: To evaluate the research ethics governance mechanisms at national level and at key healthcare institutions in Pakistan.
Methods: This pilot mapping exercise used a mixed-methods approach, involving a cross-sectional survey of 19 key healthcare research institutions, and structured in-depth interviews with the Chairs of the National Bioethics Committee and Drug Regulatory Authority of Pakistan.
Results: Eighteen institutions responded to the ethics mapping survey. Twelve public sector ERCs had a permanent structure and 17 had formal terms of reference. Seven ERCs claimed accreditation, although no central accreditation agency exists in Pakistan. Eight ERCs were chaired by the head of the institution with no fixed tenure in 13 committees, and 14 committees allowed multiple terms. Six ERCs had post-approval follow-up mechanisms, and 6 took punitive action in response to deviation from the approved protocol, or scientific misconduct. Two respondents recalled external pressures applied to committees for favourable approvals. Survey respondents mentioned lack of central research ethics guidelines as a weakness of the national governance system. Structured interviews revealed the need for formal training of members and availability of more human resources, particularly with respect to secretarial help.
Conclusion: There is a need to develop local ethical guidelines, and ensure accreditation of ERCs through the National Bioethics Committee to uphold standardization of ethics governance structure.
Keywords: ethical review committees, ethics mapping, National Bioethics Committee, accreditation, Pakistan
Citation: Jafarey AM, Shekhani SS, Raza FA, Naz S. A pilot situation analysis of mapping research ethics governance in Pakistan. East Mediterr Health J. 202x;xx(x):xxx-xxx http://doi.org/10.26719/emhj.20.xxx Received: 07/08/2021; Acceptedaccepted: 21/11/2022
Copyright: © Authors; licensee World Health Organization. EMHJ is an open access journal. All papers published in EMHJ are available under the Creative Commons Attribution Non-Commercial ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Introduction
Low- and middle-income countries often have compromised health systems and therefore require effective research ethics governance to ensure robust and ethically sound research (1, 2). Pakistan is a low- and middle-income country within the Eastern Mediterranean Region and has a disorganized health sector with limited research output (3). Pakistan has a 2-tier ethics review mechanism involving institutional and national levels. However, the quality of institutional review may vary significantly. Over the years, the number of institutional ethical review committees (ERCs) has increased to meet requirements for funding, collaboration, and publications (4). There are no verifiable data from Pakistan indicating the exact number, nature, and capacity of ERCs, and there is no accreditation process ensuring standardization. At the national level, 2 organizations are involved in ethical scrutiny of some categories of health research: the National Bioethics Committee Research Ethics Committee (NBC-REC), operational since 2004, and the Drug Regulatory Authority of Pakistan Clinical Studies Committee (DRAP-CSC), established in November 2019. The latter reviews only clinical trials, but the former provides ethical review for a wider spectrum of human research. ERC mapping exercises are useful to identify strengths and weaknesses of ethics governance systems (5–7). In Pakistan, such attempts have been sporadic and restricted to particular institutions or provinces, providing limited information (8, 9).
This current study used a mixed-methods design to conduct a systematic investigation of research governance mechanisms at key public and private research institutions in Pakistan. Additionally, it provided the first insight into national level mechanisms, examining NBC-REC and DRAP-CSC processes for their strengths, weaknesses, and overlaps. To the best of our knowledge, such an exercise has not been performed in Pakistan. This study is intended to serve as a pilot towards a nationwide mapping exercise.
Methods
Study design
This mapping exercise used a mixed-methods approach, involving a cross-sectional survey of key healthcare research institutions in Pakistan, and structured in-depth interviews with the chairs of two national regulatory bodies.
Mapping survey
A survey was developed specifically for the purpose of key institutional mapping, with 62 closed-ended questions, in 5 broad areas: (1) general information; (2) membership; (3) training for members; (4) procedure for review of proposals; and (5) challenges to review systems. Open-ended questions exploring the challenges to research ethics governance were also included. The tool was piloted on ERC members (not included in the current survey) to ensure face validity, which resulted in refinement of some questions.
The survey was administered to selected public and private sector institutes using purposive sampling. Twelve public sector institutes managed by the autonomous Pakistan Health Research Council (now known as Health Research Institute) were identified (9). One focal person from each centre was invited to participate. Three additional key public sector institutions other than the Pakistan Health Research Council were also included. Nonprobability purposive sampling was used to select 4 private sector institutions from 3 major cities because, based on our knowledge (10), these institutions produced a high volume of research, and were more suitable to provide the relevant information. Data collection took place in December 2020 and January 2021.
Structured interviews
Structured telephone interviews were conducted with the heads of NBC-REC and CSC-DRAP to better understand the workings of these organizations (11). After obtaining verbal informed consent, the interviews were audio-recorded and transcribed verbatim.
Data analysis
Data obtained from the survey were collated through surveymonkey.com. Results generated included descriptive statistics such as frequencies and percentages. Questions were grouped under themes and subthemes. Data from the survey and interviews were merged to provide an overall picture of ethical governance. Ethical approval was obtained from NBC-REC.
Results
Mapping survey
Nineteen institutes received the survey and gave a 100% response. One institution reported a nonfunctioning ERC; therefore, the data were gathered from 18 institutions with functioning ERCs: 14 from the public sector and 4 from the private sector (Table 1).
Characteristics of committees
All but 2 committees in the public sector were permanent, and 17 had formal terms of reference (publicly accessible in 10 cases). Fifteen committees conducted only ethical reviews, whereas 2 also provided scientific reviews. All committees reviewed research by staff and faculties, and 16 also reviewed student research. Nine committees accepted projects from other institutions.
Information regarding ERC procedures was available on institutional websites for 9 committees, although 17 respondents believed that this information was widely known across their institution.
Seven respondents (5 public and 2 private) declared that their committees were accredited, although none identified an actual accreditation agency. Twelve ERCs reported following published research ethical guidelines (Table 1). Nine committees (7 public and 2 private) had designated budgets and 16 had secretarial help; of which, 13 had designated secretaries and 3 had no designated staff or budget.
Leadership of ERCs
Eight ERCs (all public) were chaired by the head of the institution, 4 were headed by individuals from outside the institution, and 4 had institutional members as heads. The chair of 17 committees was appointed by the institution, and had no fixed tenure in 13 committees (10 public and 3 private). The chairs of 14 committees could serve multiple terms, with 8 serving their second term, and 2 their third and fourth terms. The other respondents either did not respond or were unaware of this factor.
Membership composition
On average, committees had 11 members (range 3–21); 13 had 6–15 members, 3 had > 16 members, and 2 had 2–5 members. Committee membership comprised medical doctors (n = 18); researchers or members from outside the institution (n = 15 each); social scientists (n = 8); lay persons (n = 6); ethicists (n = 5); and nurses, religious scholars, or lawyers (n = 4 each). All committee members were nominated by institutions. In 11 committees (9 public and 2 private), there was no fixed tenure, 4 had up to 3 years, and 3 had > 3 years. Fourteen committees had provision for multiple tenures, whereas 2 reported limiting membership to only 1 term. All committees had mechanisms to co-opt additional reviewers.
Training of members
Fourteen committees had no training prerequisite for members; 9 provided training opportunities, and in 7, prior training was not mandatory. Only 2 committees (private) that required mandatory training also funded it. Training was equally likely to be provided at the institution itself, at another institution, or online.
Process of review
The review process in different ERCs is detailed in Table 2. Respondents were asked about deviation from the usual review process. Four respondents (3 public and 1 private) believed that 2–20% of research projects from their institutions bypassed their ERC. Four believed it was because of lack of awareness of ethical requirements; 1 believed researchers wanted to cut corners, and another that researchers considered their projects free of ethical concerns. One also mentioned that researchers bypassed the ERC because they were submitting to a journal that did not require it. Provision of exemption from review was available from 12 committees. Fifteen ERCs had rejected proposals in the past, 1 had never rejected any proposal, and 2 respondents did not know. Fourteen respondents could not recall external pressure being applied to their committee to obtain approval for a research proposal. Two recalled such pressure, with 1 mentioning that the institutional head pushed for approval for a pharma-funded project.
Respondents had diverse understanding the mandate of NBC-REC (Table 3).
Follow-up of research proposals
All the committees had record-keeping and archiving mechanisms, but only 6 (4 public and 2 private) had post-approval follow-up systems in place. Six respondents (3 public and 3 private) recalled punitive actions being taken in response to deviation from protocols, or for ethical misconduct, with 8 reporting no actions. In 1 case, the penalty amounted to retraction of published work by contacting the journal concerned.
Structured interviews with heads of national regulatory bodies
NBC-REC and DRAP-CSC are permanent bodies with secretarial structures. The NBC-REC Secretariat was housed at the Pakistan Health Research Council, and DRAP-CSC at the Division of Pharmacy Services. The main role of the NBC Secretariat was to receive proposals, forward complete proposals to the REC Chair, receive the decision from the Chair, and forward it to the applicants. The secretariat also maintained the NBC website. Except for a short period during the peak of the COVID-19 pandemic, members of the secretariat were not involved in the actual review discussions. Another role played by the secretariat was coordinating with the Ministry of National Health Services Regulations and Coordination and government officials.
Membership of both committees was mostly restricted to those with a medical background, primarily physicians, although NBC-REC had elected members; all but 1 with formal qualifications in bioethics. All members of DRAP-CSC were nominated and had experience mostly in biomedical research. he NBC-REC had a mechanism for providing training to its members, but there was no such provision at DRAP-CSC.
Approved proposals required a more stringent follow-up by DRAP-CSC because it was within their mandate to halt ongoing clinical trials or disallow research at a particular site due to any. That committee received its legal regulatory powers through laws governing DRAP. The NBC-REC required researchers to submit progress reports at predetermined interviews, primarily for archiving purposes. Prior to the COVID-19 pandemic, NBC-REC reviewed proposals asynchronously via email. The Rapid Turnaround Review for COVID-19-related proposals that required a 72-hour turnaround was implemented in April 2020. Meetings were moved online and scheduled as and when proposals arrived, which necessitated several meetings a month during the first peak of the pandemic. The system worked efficiently and therefore a decision was taken to review non-COVID-19, regular proposals in virtual meetings. DRAP-CSC had monthly physical meetings but within 3 months of the onset of the pandemic, CSC also transitioned to online meetings, being held as and when required.
Challenges to research ethics governance
At the institutional level, most respondents identified deficiency of member training as a challenge to research ethics governance. Two respondents considered lack of resources for post-approval monitoring, and 1 respondent each identified conflict of interest, pressures for approval from within the institution, and negative perception of researchers towards the review process. One respondent stated that the added responsibility of reviews was burdensome on the committee members and the chair because of the added responsibility of ERC work.
Survey respondents were also asked to share perspectives regarding challenges to research ethics governance at a national level. Overall apathy towards research ethics and absence of local guidelines contributed towards weak governance structures. One respondent believed that the two national level review steps were an unnecessary duplication of effort, and the required fee payments added a financial burden. Another respondent mentioned that there was lack of coordination between institutional ERCs and national regulatory bodies. Lack of national ERC accreditation was also highlighted.
During the structured interviews, the NBC-REC Chair reported that limited secretariat help was a significant problem for the functioning of the committee. NBC-REC relied primarily on a single-person secretariat and adequate follow-up of proposals was a particular challenge. In contrast, the DRAP-CSC Chair identified no such issues.
Discussion
This study provided a systematic, albeit limited, charting of research ethics governance systems in Pakistan. Previous mapping attempts conducted in Pakistan have cited poor response rates (12). The 100% response rate in this survey can be explained by the smaller sample size and utilization of personal contacts to engage respondents. This was also the first formal account of the review role of DRAP-CSC. A previous study exploring national ethics committees in the Eastern Mediterranean Region included NBC-REC (13), while another study also provided an in-depth analysis of NBC-REC during the COVID-19 pandemic (14).
The presence of functional national regulatory bodies is promising. NBC-REC, formally notified in 2004, started with sporadic reviews in the initial years, but has since increased its review portfolio several-fold. However, the body is not without challenges, including limited administrative and secretarial support. For the most part, the secretariat has not been involved in actual meetings, leaving tasks such as minute taking to the chair.
Duplication of ethical review by NBC-REC and DRAP-CSC of proposals already reviewed within institutional committees was identified as a cause of delays. However, given the nascent field of ethics governance in Pakistan, and variability of review capacity at institutional levels, it is important that NBC-REC continues to play a central review role. The current process ensures uniformity and quality control in governance that may not otherwise be possible in Pakistan because of the lack of accreditation and regulation of ERCs.
The absence of national guidelines governing research ethics was highlighted as a deficiency. Locally adapted guidelines play a vital role in informing context-dependent governance (15). Twelve of our respondents reported following guidelines from various sources, including NBC and the Higher Education Commission. However, these institutions had no actual guidelines, which highlighted the unfamiliarity with the review processes among organizations expected to be more knowledgeable about these matters. Another important challenge identified at the national level was that while ERCs knew about NBC-REC, their responses reflected a lack of clarity regarding its mandate. If study participants occupying prominent roles in research institutions were confused, then a significantly wider lack of awareness can be assumed, which opens the way for systems to be bypassed. There is anecdotal evidence of foreign-funded studies requiring NBC-REC review being published without ever reaching the committee.
CSC-DRAP is a new development in research ethics governance, providing an enforcement arm to the regulatory framework. Its mandate is limited to reviewing and regulating clinical trials, and it has reviewed and permitted 22 mostly COVID-19-related clinical trials between November 2019 and January 2021. Some of the work of DRAP-CSC is a duplication of that of NBC-REC, but it also provides accreditation to clinical sites, contract research organizations, and physical inspections of trial sites. DRAP authorization is a prerequisite for importing and marketing of drugs in Pakistan; therefore, the organization can prevent a clinical trial from starting, or halt it in case of concerns. With barely 1 year of experience, it is too soon to infer the long-term impact of this organization.
The number of ERCs has increased over the years in Pakistan. In our study, all participating institutes except 1 had a functional committee. Nonexistence of an ERC within the Pakistan Health Research Council umbrella is a matter of concern, reflecting a possible lack of research at that institution. That particular institution was located in an underdeveloped province with poor health indicators and low research output.
The trend towards an increase in the number of institutional ERCs reflected heightened awareness for such a need. However, these committees often only exist on paper, and may not conduct rigorous review of research projects (16, 17). The increasing number of ERCs could be in response to regulators such as Higher Education Commission, College of Physicians and Surgeons Pakistan, and Pakistan Medical Commission requiring physicians to publish research in order to qualify for fellowships or secure promotions, rather than a desire to observe ethical norms during research (18, 19).
Institutional commitment to ethical review governance is critical for successful functioning of committees, and is reflected in support provided through budgets, secretarial help, and training opportunities. In this survey, while most committees had some secretarial help, only 9 had budgetary allocations. A well-functioning secretariat assists in the running of meetings and ensures steady communication with applicants and proper post-approval follow-up, and requires funding (20). ERCs now require members to obtain formal certification in research ethics (21, 22). However, only 2 ERCs in this study had any training requirements. This is concerning because most committee members would have had no relevant training in their professional education.
Our survey illustrated a diverse membership in most committees. It was unsurprising that most committees primarily had physicians as members because the survey covered only medical institutions; however, it was encouraging to note that most committees also had external representation. This added diversity and led to unrestricted discussions, with external members generally being more forthcoming with critical comments (23). Only 4 committees had nurses, which, while expected because of their marginalized status in the medical hierarchy, was disappointing because their exclusion resulted in the loss of important perspectives (24). Although lay person and community representation on committees is recommended, only 6 committees in our survey had such representation. This can be explained by the exclusionary medical culture in Pakistan. An encouraging finding was the inclusion of social sciences representation on 9 committees. This reflected a move of committees beyond their traditional comfort zone of reviewing biomedical research, and an enhanced ability to review public health and social sciences research. It was noteworthy that 5 committees had ethicists as members, implying that few committee members may have received formal bioethics training.
Type and length of tenure for members and chairs emerged as a concern. Membership and chairpersonship were entirely by nomination, which may have limited committee membership to older people, given the hierarchical culture in Pakistan. The lack of fixed tenure indicated potential stagnation. The ERC being chaired by the head of the same institution reflected a potential conflict of interest. While this was an accepted trend noted in an unpublished study in 2010, the current survey showed that 8 of the 18 committees were chaired by their institutional heads (25, 26). Our sample was limited, but it was alarming to see that institutions were unaware of or were ignoring this potential conflict of interest.
Our effort to present a realistic snapshot of research ethics governance in Pakistan had some limitations. The survey covered only selected institutions, and the interviews only captured the perceptions of the chairs of the 2 national committees, and not the members, whose views could add valuable insight.
Conclusion
Our survey indicated variation in the type and quality of review at the institutional level, which was a reflection of the working of the ERCs. Accreditation of all ERCs through the NBC could ensure uniformity, quality control, and stronger cohesion between national and institutional ethics governance systems. The study also pointed towards the need to have a comprehensive, countrywide mapping of research and ethics regulatory capacity. Additionally, there is a need for national research ethics guidelines. A local, relevant guidance document would be important to provide a framework for ethical conduct, especially with the growth of research in Pakistan.
References
1. Kebede D, Zielinski C, Mbondji PE, Sanou I, Kouvividila W, Lusamba-Dikassa PS. Research and its governance in health research institutions in sub-Saharan African countries: results of a questionnaire-based survey. J R Soc Med. 2014 May; 107(1_suppl):55–69. https//doi.10.1177/0141076814531751 PMID:24914129
2. Akhtar T, Khan J. Health research capacity in Pakistan. Pakistan: Council on Health Research for Development; 2000 (http://www.cohred.org/downloads/681.pdf, accessed 13 March 2023).
3. Jafarey AM, Iqbal SP, Hassan M. Ethical review in Pakistan: the credibility gap. J Pak Med Assoc. 2012 Dec; 62(12);1354–7. PMID: 23866494
4. Hofman KJ, Kanyengo CW, Rapp BA, Kotzin S. Mapping the health research landscape in Sub-Saharan Africa: a study of trends in biomedical publications. J Med Libr Assoc. 2009 Jan; 97(1):41–4. http://doi.org/10.3163/1536-5050.97.1.007 PMID:19158994
5. Dobrow MJ, Costa S, Israr S, Chafe R. Mapping health services and policy research settings in Canada: following the money, the publications and the interest. Healthc Policy. 2010 Nov; 6(2):84. PMID:22043225
6. Clarke A, Gatineau M, Grimaud O, Royer-Devaux S, Wyn-Roberts N, Le Bis I, et al. A bibliometric overview of public health research in Europe. Eur J Public Health. 2007 Jan 1; 17(Suppl 1):43-9. https://doi.org/10.1093/eurpub/ckm063 PMID:17666422
7. Abrar S, Ronis KA, Khan S, Siraj S, Safdar W, Khalid Y et al. Status of ethical review boards at all medical colleges of Khyber Pakhtunkhwa. J Ayub Med Coll Abbottabad. 2015 Jun 20; 27(2):411–4. PMID:26411130
8. Ahsin S, Saeed GN. Self-evaluation of ethical review committee’s functioning at Foundation University Medical College (FUMC) through structured constitution-practice outcome (CPO) assessment model. J Pak Med Assoc. 2017 Jan 1; 67:42. PMID:28065953
9. Health Research Institute. HRI Research Institute. Available at: https://hri.nih.org.pk/research-centres.html
10. Etikan I, Musa SA, Alkassim RS. Comparison of convenience sampling and purposive sampling. Am J Theoret Appl Stat. 2016 Jan 5;5(1):1–4. https://doi.org/10.11648/j.ajtas.20160501.11
11. Lindlof TR, Taylor TR. Qualitative communication research methods, 2nd edition. Thousand Oaks: Sage; 2002.
12. Salim M, Hamid S. Independent review of research proposals from ethical point of view in Pakistan. J Ayub Med Coll Abbottabad. 2018 Oct 1;30(4):588-91. PMID: 30632343
13. Abou-Zeid A, Afzal M, Silverman HJ. Capacity mapping of national ethics committees in the Eastern Mediterranean Region. BMC Med Ethics. 2009 Dec;10(1):1–7. https://doi.org/10.1186/1472-6939-10-8 PMID:19575813
14. Shekhani SS, Iqbal SP, Jafarey AM. Adapting the ethical review process for COVID-19 research: reviewers’ perspectives from Pakistan. East Mediterr Health J. 2021 Dec 1;27(11):1045–51. https://doi.org/10.26719/emhj.21.053 PMID:34927707
15. Kumar NK. Bioethics activities in India. East Mediterr Health J. 2006;12(Suppl 1):S56–65. PMID:17037690
16. Gilman RH, Garcia HH. Ethics review procedures for research in developing countries: a basic presumption of guilt. CMAJ. 2004 Aug 3;171(3):248–9. https://doi.org/10.1503/cmaj.1031121 PMID:15289424
17. Moazam F. Research and developing countries: hopes and hypes. East Mediterr Health J. 2006; 12 (Suppl 1):S30–6. PMID:17037686
18. HEC to facilitate university faculty only under rules, regulations: chief. Daily Times. 21 October 2020. (https://dailytimes.com.pk/680039/hec-to-facilitate-university-faculty-only-under-rules-regulations-chief/, accessed 13 March 2023).
19. Memon AR. Research publications and education in Pakistani medical universities: Avoiding predatory journals and improving the quality of research. J Pak Med Assoc. 2017 Jun 1; 67(6):830–3. PMID:28585576
20. Peteris Z. Bioethics networks: collaborating across national boundaries. Bioethics and shared values of Europe. In: National bioethics committees in action. Paris: United Nations Educational, Scientific and Cultural Organization; 2010:105–7.
21. The WHO strategy on research for health. Geneva: World Health Organization; 2012 (https://www.afro.who.int/sites/default/files/2020-11/WHO_Strategy_on_research_for_health.pdf, accessed 13 March 2023).
22. Davies H, Wells F, Druml C. How can we provide effective training for research ethics committee members? A European assessment. J Med Ethics. 2008 Apr 1;34(4):301–2. http://dx.doi.org/10.1136/jme.2007.021485 PMID:18375685
23. Research ethics committees: basic concepts for capacity-building. Geneva: World Health Organization; 2009 (https://apps.who.int/iris/handle/10665/44108, accessed 13 March 2023).
24. Savage TA. The nurse’s role on ethics committees and as an ethics consultant. Semin Nurse Manage 1994 Mar 1;2(1):41–7. PMID:7922649
25. Jafarey AM. Conflict of interest issues in informed consent for research on human subjects: a South Asian perspective. Sci Eng Ethics. 2002 Sep;8(3):353–62. https://doi.org/10.1007/s11948-002-0055-9 PMID:12353363
26. Sheikh AL. Pharmaceutical research: paradox, challenge or dilemma? East Mediterr Health J. 2006; 12(Suppl 1):S42–9. PMID:17037688
A randomized controlled trial on the dietary intake of Saudi Arabian female adolescents living in Arar
Abeer Bahathig1,2 and Hazizi Abu Saad1
1Department of Nutrition, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Selangor, Malaysia.
2Department of Nutrition & Food Science, College of Home Economics, Northern Border University, Arar, Saudi Arabia (Correspondence to Hazizi Abu Saad:
Abstract
Background: Lifestyle changes in Saudi Arabia have affected the dietary intake of adolescents: they now consume more unhealthy foods.
Aim: We used a randomized controlled trial to assess the dietary intake of Saudi Arabian female adolescents living in Arar.
Method: In this randomised cluster study, female students were selected randomly from assigned schools to form the intervention group (n = 68) and control group (n = 70). Initially, a 60-minute seminar was held for the intervention group mothers. Six 90-minute sessions were arranged for the intervention group on topics such as food groups, healthy and unhealthy eating, body image and physical activity were delivered over 3 months. The data were analysed using generalized estimating equations.
Results: The interaction effect (group by time) between the groups revealed statistically significant differences for dairy products (P < 0.001), sweetened beverages (P < 0.001), sweetened baked goods (P = 0.022) and fruits and vegetables (P < 0.003). The intervention significantly increased the intake of dairy products (P < 0.001) and fruits and vegetables (P = 0.003), and reduced that of sweetened beverages (P < 0.001) and sweetened baked goods (P = 0.010) in the intervention group.
Conclusion: This intervention exerted a greater positive effect on the intervention group than the control group regarding dairy products, sweetened beverages and fruits and vegetables. No significant difference was found in intake of energy and macronutrients between the groups (P > 0.05).
Keywords: nutrition intervention, dietary intake, fruits and vegetables, school students
Citation: Bahathig AA, Abu Saad H. A randomized controlled trial on the dietary intake of Saudi Arabian female adolescents living in Arar. East Mediterr Health J. 2020;26(x):xxx–xxx. https://doi.org/10.26719/emhj.XXXX Received: 04/09/2022; accepted: 22/12/2022
Copyright © Authors 2023; Licensee: World Health Organization. EMHJ is an open access journal. This paper is available under the Creative Commons Attribution Non-Commercial ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Introduction
A healthy diet, i.e. not consuming large quantities of sugar, salt and fat, can protect the body from obesity, heart disease, diabetes and cancer (1,2). Adolescents need to consume adequate amounts of nutrients for growth, and the recommended daily intake of essential vitamins and minerals can be met if healthy foods, e.g. fruits and vegetables, are consumed daily (3).
The World Health Organization (WHO) recommends a daily diet comprising a variety of food groups (2), but adolescents from the Eastern Mediterranean region do not follow these recommendations and consume large amounts of fats (including saturated fats) and sugary drinks, and few vegetables, fruits and other high-fibre foods (4). Altogether, 25% of Saudi adolescents and adults eat french fries at least 3 times week, and more than 50% consume chocolates and sweets (5). It has been reported that Saudi Arabian adolescents aged 12–16 years consume sugary foods at a rate of 11.7 (standard deviation 2.2) (6). The Healthy Food Palm was developed around Saudi culture and eating habits, and emphasizes consumption based on food groups with the aim of enhancing nutrient adequacy and improving health (7). Nevertheless, a 2021 cross-sectional study on students from Al-Ahsa, Saudi Arabia, reported that 49.5% of normal weight and 100% of obese participants drank soft drinks daily (8).
Many Saudi Arabians do not follow Ministry of Health guidelines strictly: around 48% of adolescents need nutrition counselling to establish healthy eating behaviours (9). Nutrition education at an early age can influence the adoption of healthy eating habits before the development of unhealthy habits, and those with healthy habits are more likely to maintain these over time (10). Theory-based nutrition intervention emphasising the provision of information and learning skills to practise new behaviours can be effective for dietary change (11). A 2021 randomized cluster trial in India significantly changed dietary practices (P = 0.030) and consumption of junk food (P = 0.003) among adolescents (12). School students in Lebanon significantly increased their intake of fruits and vegetables and reduced their consumption of crisps after a 3-month nutritional intervention compared with the control group (P < 0.050) (13). In a Saudi Arabian study, adolescents in the intervention group showed greater changes in their consumption of healthy (vegetables and fruits) and unhealthy (desserts and snacks) foods compared with the control group after a 9-week school-based nutrition intervention (14).
Our study was conducted to assess dietary intake of Saudi Arabian female adolescents living in Arar.
Methods
Design
This single blinded randomized cluster study was conducted (January–June 2020) among females from 2 government intermediate schools. Healthy Saudi Arabian female adolescents aged 13–14 years agreed to participate in the study with the consent of either parent. Adolescents who had disabilities or noncommunicable diseases were excluded.
Rosner’s formula was used to determine the sample size according to the mean difference scores for nutritional knowledge in both groups (15,16). A minimum of 68 participants was required for each group, with 15% added to cover dropouts (17). Therefore, a total of 160 participants was required, i.e. 80 participants per group. The selection and assignments of the participants were implemented randomly using Excel software. We selected 40 participants aged 13 years and 40 aged 14 years for each group. The intervention aims and procedures were explained to the participants and their parents before consent forms were collected.
Ethical approval
Before this intervention study began, we received approval from the ethics committee for research involving human subjects, Selangor, Malaysia JKEUPM (Reference No: UPM/TNCPI/RMC/JKEUPM/1.4.18.2), and the local committee on bioethics (Reference No. 13/40/H; HAP-09-A-043), and the Ministry of Education in Arar City, Saudi Arabia.
Nutrition intervention on dietary intake
This school-based nutrition intervention was developed for females aged 13–14 years, focussing on knowledge and skills based on social cognitive theory to improve the dietary intake behaviours of adolescents (18). Initially, the first researcher conducted a 60-minute seminar for the mothers of the intervention group at the school to encourage their adolescents to eat healthily, be happy with their body image and get 60 minutes of physical activity daily. For the adolescents in the intervention group, 6 interactive 90-minute sessions, 5 of which specifically focussed on nutrition, were delivered fortnightly for 3 months during school hours. Figure 1 showed the methodology flowchart for the nutrition intervention.
The intervention participants were divided into 2 groups based on classroom size. Topics covered the importance of eating healthily and following the Saudi food guide pyramid regarding weight status, food groups, serving size and variety, macronutrients, consuming main meals on time daily, reading food labels, the drawbacks of consuming unhealthy foods (i.e. those high in sugar, fat and salt, such as fast food, soft drinks and other sweetened beverages), type 2 diabetes and obesity, the importance of positive body image and physical activity.
The sessions comprised 2 parts, one on knowledge and the other on activities that apply the knowledge. Booklets, PowerPoint presentations, whiteboards, group discussions, flashcards and games were used, with awards given out to both groups during data collection. The control group received the traditional education, but was provided with the intervention materials upon the completion of the study. All educational sessions were developed based on the preliminary study; guidance from nutrition experts at the Nutrition and Food Sciences, Medicine and Applied Medical Sciences Departments in Arar; and previous studies (14,16,19–21). The intervention was pilot tested before implementation of the actual intervention to ensure accuracy and appropriateness.
A total of 3 face-to-face survey questionnaires were completed to collect data:
• at baseline,
• immediately after the 3-months intervention ended,
• at the 3-month post-intervention follow up.
Participants and their parents signed consent forms before data collection. The questionnaire covered sociodemographic characteristics (participants’ age, parents’ education level, number in household, number of siblings, monthly income, etc.) and dietary intake.
Food intake
The average scores for consumption of food groups, macronutrients and dietary energy were obtained through a semi-quantitative food frequency questionnaire and 24-hour recall for 3 days using the ProDiet Analysis, version 6 (Axxya Systems; Redmond, Washington (https://nexgen1.nutritionistpro.com/shop/product-detail/nutritionist-pro-diet-analysis-software-13. New Saudi Arabian food recipes were added to the US Department of Agriculture’s database, which was used in the study (22). Estimated food intake was derived from frequency of consumption for the food groups, nutrients and energy intake using a conversion factor (23):
amount of food consumed (g/day) = frequency of intake × serving size × total number of servings × weight of food in one serving (24).
High-frequency consumption would be once or more a week, and low frequency would be less than once (25). If consumption frequency was high, the score should be ≥ 0.5 (26).
Food habits
Almajwal et al. developed a semi-quantitative food frequency questionnaire covering 74 food items for the Saudi Arabian adolescent population (27). The average agreement for food groups was 70.9%, with 70.1% for nutrients. It was categorized as times/day (1, 2–3, 4–5 and 6–7), times/week (1–2, 3–4 and 5–6), times/month (1 and 2–3) and never. The food group components comprised cereals and grains (7 items, e.g. popcorn and bread); meat products (11 items, e.g. eggs, fish and chicken); mixed dishes (12 items, e.g. chicken burger and pizza); dairy products (6 items, e.g. full fat yoghurt and cream cheese); sweetened beverages (9 items, e.g. concentrated orange juice and grape juice); sweet and baked goods (13 items, e.g. honey and cake); and fruits and vegetables (16 items, e.g. dates and tomato) (27). Each participant was asked to report on their food consumption in terms of amount and serving size.
24-hour diet recall
To gather more information, 24-hour diet recalls were performed 3 times during the previous week: 2 on weekdays and one at the weekend. Each participant’s food and drink activity was collected to assess energy and macronutrient intake, taking into account time of intake and how food was prepared.
Statistical analyses
We analysed the data using SPSS; P-value was < 0.05. The Mann–Whitney U-test and the chi-squared test were used to ensure the demographic homogeneity of the variables within and between groups at baseline. The data were not normally distributed and we used a randomized cluster design, so the generalized estimating equations test was used to compare mean scores for the food group, energy and macronutrient intakes within and between groups using an autoregressive correlation structure. The group by time effect interaction was applied to determine the effectiveness of the nutrition intervention on dietary intake at 3 different times.
Results
Sociodemographic characteristics
Dropout rates of 5.3% (n = 4) and 6.5% (n = 5) were registered immediately, and 10.6% (n = 8) and 10.3% (n = 8) at follow-up for the intervention group and control group respectively. Various reasons were cited, e.g. too busy, left the area, or could not complete the 3+ sessions. Ultimately, 89.4% (n = 68) of the intervention group and 89.7% (n = 70) of the control group finished the study.
Comparing sociodemographic variables between the 2 groups, significant differences were found only for monthly income (P = 0.005) and number of siblings (P = 0.045), which were viewed as covariates. No significant differences were found for age, parents’ education level and number (of inhabitants) per household, i.e. family size (P > 0.050).
The effectiveness of the nutrition intervention on intake frequency of food groups, energy and macronutrients
A generalized estimating equations test was conducted to gauge intake frequency of the 7 food groups, energy and macronutrients. Significant differences were found in the mean scores at baseline between the groups for cereals and grains; fish, poultry and red meat; and sweetened beverages. Thus, these variables were viewed as covariates during the analysis. Table 1 shows the intake frequencies of both groups for the food groups, energy and macronutrients.
When the baseline score for intake of cereals and grains was excluded, the effect on the groups was statistically significant (P < 0.001), whereas no significant effect was reported for intake of cereals and grains (P = 0.370). The interaction time (group by time) effect was statistically significant for intake of dairy products (P < 0.001), sweetened beverages (P < 0.001), sweetened baked goods (P = 0.022) and fruits and vegetables (P = 0.003), indicating that the intervention group and the control group did not experience the same trend over the 3-month study period. We observed no statistically significant improvement in the consumption of fish, poultry and meat (P = 0.625) and mixed dishes (P = 0.174), i.e. both groups shared the same pattern for these over the study period. Intake of cereals and grains, dairy products and fruits and vegetables increased while intake of sweetened beverages and sweetened baked goods decreased. For intake frequencies for energy and macronutrients, the interaction time (group by time) effect on total dietary energy was not statistically significant (P = 0.210) (Table 2). Thus, both groups demonstrated the same pattern. The interaction time (group by time) effect was not significant for carbohydrates (P = 0.382), protein (P = 0.361) and fat (P = 0.452) during the study period.
The Bonferroni post hoc test was conducted on both groups of participants to determine their differences in intake frequencies for food groups, energy and macronutrients over time (Table 3). The results indicated significant differences in intake frequencies for dairy products (P < 0.001), sweetened beverages (P < 0.001), sweetened baked goods (P 0.050), in which the time effect was classed as small (d < 0.50). Moreover, no significant differences were found between the intervention and control groups throughout the study period for total intake of energy and macronutrients from the diet (P = 1.000) except for dietary energy at baseline and immediately after the intervention in the control group. The time effect was small for energy and macronutrient intake (d < 0.50) between the groups during the study period.
Statistically significant changes were found between the groups during the follow-up tests in regard to the intake frequencies of dairy products, sweetened beverages and fruits and vegetables (P 0.050). Intake frequency for cereals and grains for both groups was significant at baseline and immediately after the intervention (P 0.050) over time. The effect size between the intervention group and control group during the study period was small for intake of dietary energy and macronutrients (d < 0.50) (Table 4).
Discussion
Dietary patterns are shifting with increasing urbanization, lifestyle changes and increased production of processed foods with people currently consuming greater quantities of sodium, sugar, fat and salt. Consumption of cooking oil and salt is now double the recommended daily intake. However, many people consume inadequate amounts of fibre, fruits and vegetables (1). This unhealthy eating behaviour can lead to noncommunicable diseases and obesity (2). A seminar was held for mothers to encourage their daughters to replace their intake of unhealthy food with healthy food. The sessions on nutrition information and healthy activities, which were held for the intervention group, were effective in eliciting positive improvements regarding intake of the food groups except for intake of cereals and grains; fish, poultry and red meat; and mixed dishes. This might indicate that more information should be provided to adolescents about these food groups.
Saha et al. reported that after 6 nutritional sessions, students increased their intake of fruits and vegetables immediately (P < 0.001) (18). The outcomes matched those of Salem and Said, who included nutritional sessions (29). Girls ages 12–15 increased their consumption of healthy food, particularly fruits and vegetables (P < 0.001). The reduced consumption of salt, sugar, unhealthy drinks and foods was maintained (P < 0.001). Similarly, in a study on Syrian refugees in Lebanon, an increase in healthy food consumption was found among adolescents in the intervention group (e.g. fruits and vegetables) along with a reduction in intake of unhealthy food (e.g. sweets and fast food) immediately after a 3-month intervention (16). Previously, intervention group participants might have increased their dietary intake due to improved knowledge. Thus, the components and instruments were appropriate for the participants to achieve their targeted goals (30).
Fetohy et al. (31) conducted 3 sessions among Saudi Arabian intermediate and secondary students, who subsequently increased their daily consumption of fresh food (P = 0.050) and whole grains (P = 0.020) and avoided fatty meals (P = 0.030). However, our participants did not demonstrate any significant changes in their intake of cereals and grains. Previous research conducted among Saudi Arabian adolescents found significant improvement in the consumption of healthy snacks and food groups as well as a reduction in the consumption of unhealthy foods, e.g. soft drinks, fast food, chocolates and other sweets (P < 0.050) (20). The participants were younger than those involved in the present intervention, so their health concerns were less important than the older participants in the present study (32).
Our findings were attributed to the nutrition intervention offered to participants as a way to reduce unhealthy eating. Although consumption of cereals and grains; fish, poultry and red meat; and mixed dishes did not improve among intervention group participants, significant differences were found in their intake of dairy products, sweetened beverages, sweetened baked goods and fruits and vegetables based on social cognitive theory. Furthermore, this school-based intervention provided activities, e.g. planning healthy main meals based on a variety of foods and serving sizes, recognising healthy vs unhealthy foods, reading food labels (calories, carbohydrates, serving size) and discussing with the group how to replace unhealthy eating with healthy eating behaviours. These activities may have helped the present participants acquire and maintain new healthy eating behaviours. Recent research has suggested that nutrition education sessions be conducted among Saudi Arabian, Lebanese and Egyptian participants, with an emphasis on group discussions about food groups and the importance of eating healthy food (13,14,29). Information can be provided through PowerPoint presentations to encourage participants to eat healthy foods and reduce their consumption of unhealthy foods. The appropriate components, activity tools and duration of an intervention all play a vital role in its success (30).
This study had certain limitations. Only females were included because they are taught in schools separate from males in Saudi Arabia. The investigation was conducted in a single intervention school and city, thereby preventing generalization of the results to all 13- and 14-year-old Saudi Arabian females. Parental influence on changing intake frequencies for food groups among participants was not examined. This study was developed after determining the need for a nutrition education intervention among the adolescent female population.
This school-based intervention included sessions and activities that enhanced nutrition intervention and built new behaviours among participants. To avoid bias, 24-hour recall was used for the 3 days when the data were being collected.
Conclusion
This intervention was successful at increasing dietary intake of dairy products and fruits and vegetables, and reducing intake of sweetened beverages and sweetened baked goods among the intervention group participants. It is recommended that further studies apply this nutrition intervention to dietary intake among young Saudi Arabian females, both above and below age 13–14 years, after adjusting estimates to cover the entire adolescence period and to protect them from obesity and disease. The number of schools and cities in the intervention should be expanded to determine the effectiveness of this nutrition intervention among Saudi Arabian female adolescents. We recommend that parents participate in the sessions to accurately determine the effect of the intervention. Future researchers should focus more on energy and macronutrients to elicit changes among adolescents.
Funding: None.
Competing interests: None declared.
References
1. Adolescent health. Geneva: World Health Organization; 2022 (https://www.who.int/health-topics/adolescent-health#tab=tab_1, accessed 10 July 2022).
2. Healthy diet. Geneva: World Health Organization; 2020 (https://www.who.int/news-room/fact-sheets/detail/healthy-diet, accessed 25 March 2023).
3. State indicator report on fruits and vegetables. Atlanta: Centers for Disease Control and Prevention; 2018. (https://www.cdc.gov/nutrition/downloads/fruits-vegetables/2018/2018-fruit-vegetable-report-508.pdf, accessed 25 March 2023).
4. Al-Jawaldeh A, Taktouk M, Nasreddine L. Food consumption patterns and nutrient intakes of children and adolescents in the Eastern Mediterranean region: a call for policy action. Nutrients. 2020;12(11):3345. doi:10.3390/nu12113345
5. Alzamil HA, Alhakbany MA, Alfadda NA, Almusallam SM, Al-Hazzaa HM. A profile of physical activity, sedentary behaviors, sleep, and dietary habits of Saudi college female students. J Fam Community Med. 2019;26(1):1–8. doi:10.4103/jfcm.JFCM_58_18
6. Alkhaldi AK, Alshiddi H, Aljubair M, Alzahrani S, Alkhaldi A, Al-Khalifa KS, et al. Sex differences in oral health and the consumption of sugary diets in a Saudi Arabian population. Patient Prefer Adherence. 2021;15:1121–1131. doi:10.2147/PPA.S308008
7. Halawani R, Jaceldo-Siegl K, Bahjri K, Heskey C. Saudi population’s adherence to the Healthy Food Palm: a cross-sectional study. FASEB J. 2019;33(Suppl. 1):755.4. doi:10.1096/fasebj.2019.33.1_supplement.755.4
8. Jouhar R, Ahmed MA, Khurshid Z, Bokhari SAH. Association of BMI, diet, physical activity, and oral hygiene practices with DMFT index of male dental students at King Faisal University, Al-Ahsa. Nutrients 2021;13(1):224. doi:10.3390/nu13010224
9. Almuhlafi M, Jamilah KA, Almutairi AF, Salam M. Relationship between early menarche, obesity, and disordered eating behaviors: A school-based cross-sectional survey in Northern Saudi Arabia. Diabetes Metab Syndr Obes. 2018;11:743–51. doi:10.2147/DMSO.S180697
10. Contento IR. Nutrition education: linking research, theory, and practice, 3rd ed. Burlington, MA: Jones & Bartlett Learning; 2016.
11. Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs: Prentice-Hall, Inc; 1986.
12. Mahajan A, Negi PC, Gandhi S, Sharma D, Grover N. Impact of school-based health behavioral intervention on awareness, practice pattern of healthy lifestyle, and cardiometabolic risk factors among school children of Shimla: a cluster-randomized, intervention study. Indian J Pediatr. 2021; 89(4):343–350. doi:10.1007/s12098-021-03786-6
13. Habib-Mourad C, Ghandour LA, Maliha C, Awada N, Dagher M, Hwalla N. Impact of a one-year school-based teacher-implemented nutrition and physical activity intervention: main findings and future recommendations. BMC Public Health. 2020;20:256. doi:10.1186/s12889-020-8351-3
14. Hefni D. Assessing the effectiveness of an obesity-prevention intervention to improve healthy lifestyle among Saudi school girls aged 9 to 16: a feasibility study [thesis]. University of Salford, United Kingdom; 2017.
15. Rosner B. Fundamentals of biostatistics, 8th ed. Boston: Cengage Learning; 2015.
16. El Harake MD, Diab M, Kharroubi S, Hamadeh SK, Jomaa L. Impact of a pilot school-based nutrition intervention on dietary knowledge, attitudes, behavior and nutritional status of Syrian refugee children in the Bekaa, Lebanon. Nutrients. 2018;10(7):913. doi:10.3390/nu10070913
17. Cramer H, Haller H, Dobos G, Lauche R. A systematic review and meta-analysis estimating the expected dropout rates in randomized controlled trials on yoga interventions. Evid Based Complementary Altern Med. 2016;2016:1–7. doi:10.1155/2016/5859729
18. Saha S, Dawson J, Murimi M, Dodd S, Oldewage-Theron W. Effects of a nutrition education intervention on fruit and vegetable consumption-related dietary behavioural factors among elementary school children. Health Educ J. 2020;79(8):963–73. doi:10.1177/0017896920944421
19. Ahmad Bahathig A, Abu Saad H, Md Yusop NB, Mohd Shukri NH, El-Din MME. Relationship between physical activity, sedentary behavior, and anthropometric measurements among Saudi female adolescents: a cross-sectional study. Int J Environ Res Public Health. 2021;18(16):8461. doi:10.3390/ijerph18168461
20. Alamri E. Factors affecting food intake and the role of nutrition education in the understanding and implementation of healthy dietary habits in Saudi adolescent girls [thesis]. University of Plymouth, United Kingdom; 2017.
21. Sharif Ishak IZS, Chin YS, Taib MNM, Shariff ZM. School-based intervention to prevent overweight and disordered eating in secondary school Malaysian adolescents: A study protocol. BMC Public Health. 2016;16(1):1101. doi:10.1186/s12889-016-3773-7
22. Al-Feda AA. Nutritional assessment guide for the Kingdom of Saudi Arabia. Riyadh: King Fahd National Library; 2018.
23. Norimah Jr AK, Safiah M, Jamal K, Haslinda S, Zuhaida H, Rohida S, et al. Food consumption patterns: findings from the Malaysian adult nutrition survey (MANS). Malays J Nutr. 2008;14(1):25–39.
24. Wessex Institute of Public Health Medicine. Software Package for food frequency questionnaire. University of Southampton; Highfield; 1995.
25. Adams J, Goffe L, Brown T, Lake AA, Summerbell C, White M, et al. Frequency and socio-demographic correlates of eating meals out and take-away meals at home: cross-sectional analysis of the UK national diet and nutrition survey, waves 1–4 (2008–12). Int J Behav Nutr Phys Act. 2015;12:52. doi:10.1186/s12966-015-0210-8
26. Hackshaw A. A concise guide to observational studies in healthcare. London: John Wiley & Sons, Ltd; 2014.
27. Almajwal A, Abulmeaty M, Alam I, Razzak S, Alqahtani A. Development of food frequency questionnaire (FFQ) for the assessment of dietary intake among overweight and obese Saudi young children. Nutrire. 2018;43(1):29. doi:10.1186/s41110-018-0088-8
28. Cohen J. Statistical power analysis for the behavioral sciences (2nd ed.). New York: Academic Press, Inc; 2013.
29. Salem GM, Said RM. Effect of health belief model based nutrition education on dietary habits of secondary school adolescent girls in Sharkia governorate. Egypt J Commun Med. 2018;36(3):35–47.
30. Fertman CI, Allensworth DD. Health promotion programs: From theory to practice, 2nd ed. San Francisco: Jossey-Bass A Wiley Imprint; 2016.
31. Fetohy EM, Mahboub SM, Abusaleh HH. The effect of an educational intervention on knowledge, attitude and behavior about healthy dietary habits among adolescent females. J High Inst Public Health. 2020;50(2):106–12.
32. Hashemian M, Abdolkarimi M, Asadollahi Z, Nasirzadeh M. Effect of “social cognitive theory”-based intervention on promoting physical activity in female high-school students of Rafsanjan city, Iran. J Educ Community Health. 2021;8(2):111–9. doi:10.52547/jech.8.2.111
How medical waste management was affected by the COVID-19 pandemic in hospitals
Fusun Z. Akcam,1
1Department of Infectious Diseases and Clinical Microbiology, Faculty of Medicine; 2Department of Environmental Engineering Faculty of Engineering; 3Department of Public Health, Faculty of Medicine, Suleyman Demirel University, Isparta, Turkey (Correspondence to Fusun Z. Akcam:
Abstract
Background: The COVID-19 pandemic has led to an increase in the production of medical waste in hospitals.
Aim: To evaluate how the COVID-19 pandemic is affecting medical waste management in hospitals.
Methods: Three different types of hospital in Isparta Province, south-western Turkey, were selected to examine their medical waste production. The number of patients, amount of medical waste and occupancy rates of the 3 hospitals in the pre-pandemic (2019–2020) and pandemic (2020–2021) periods were compared within themselves and with each other. The data were analysed using SPSS, version 22.0, and statistical significance was set at P < 0.05.
Results: During the pandemic period, the number of inpatients in both public hospitals decreased, while the number in the private hospital increased. The amount of medical waste in the pre-pandemic period was 8.4 kg/person in the state hospital, 7.7 kg/person in the university hospital and 6.3 kg/person in the private hospital. In the pandemic period, these amounts were 14.2, 10.1 and 7.6 kg/person, respectively.
Conclusion: There was a significant increase in medical waste production during the COVID-19 pandemic. It would be appropriate for health institutions to review their medical waste management.
Keywords: medical waste, hospital waste management, personal protective equipment, COVID-19
Citation: Akcam FZ, Pamukoglu Y, Uskun E. How medical waste management was affected by the COVID-19 pandemic in hospitals. East Mediterr Health J. 2023;29(7):xxx–xxx. https://doi.org/10.26729/emhj.23.068
Received: 05/06/22; accepted: 22/12/22
Copyright © Authors 2023; Licensee: World Health Organization. EMHJ is an open access journal. This paper is available under the Creative Commons Attribution Non-Commercial ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Introduction
Certain types of waste are produced as a consequence of manufacture and services in every service-generating institution. Some of these wastes are harmful to human life and may disrupt the ecological balance by staying in the air, water and land for a long time. Special measures need to be taken regarding the transportation, storage and disposal of these waste materials.
Waste materials containing infective disease-causing pathogens are defined as infectious health care-related wastes, and include blood and other bodily fluids, laboratory cultures and materials contaminated with infectious matter (1,2). Safe and environmentally conscious management of these wastes will prevent their negative impact on health and the environment, and thus protect public health.
In the fight against COVID-19, the management of medical, domestic and other hazardous wastes is an urgent and fundamental public service to minimize possible effects on health and the environment. Contaminated wastes such as masks, gloves and other protective equipment and numerous noncontaminated medical and hazardous wastes emerged during the pandemic (3)
A number of studies have been conducted on the investigation of medical and solid wastes, particularly during the pre- and post-pandemic eras (4,5), and hospital-based studies are frequently carried out (6–9). The aim of this study was to evaluate how COVID-19 pandemic affected medical waste production in 3 types of hospital.
Methods
Data collection
Three hospitals in Isparta Province, south-western Turkey, were selected for this study: a university hospital, a state hospital and a private hospital. Isparta is close to Antalya, one of the most important tourism centres in Europe and Turkey. We reviewed data on medical waste production and management from the 3 hospitals during the first year of the COVID-19 pandemic and the previous year. On receiving approval from the Provincial Directorate of Health, data on the number of patients and rates of medical waste and occupancy were retrieved from the hospital records. The state hospital is the largest hospital in the province with an 830-bed capacity. The research and training hospital at the university has a 595-bed capacity. Among the private hospitals, the largest one, having a 260-bed capacity, was selected.
For this study, the pre-pandemic period was considered to be between 1 April 2019 and 31 March 2020 and the pandemic period between 1 April 2020 (the date when the first COVID-19 patient was hospitalized in our hospitals) and 31 March 2021. The number of patients, amount of medical waste and occupancy rates in both periods were compared.
Statistical analysis
We used the Kolmogorov–Smirnov test to test for the conformity of the variables to the normal distribution, taking into account skewness and kurtosis indices. Conformity to the normal distribution was set at P > 0.05 for comparisons of the Kolmogorov–Smirnov test and where skewness and kurtosis index values were less than 2 times the standard error. Data were presented as descriptive statistics (mean, standard deviation) and by analysing using hypothesis testing; parametric tests were used in all hypothesis tests. The Pearson test was used to assess all correlations between number of patients, occupancy rate and amount of medical waste. We used 1-way analysis of variance (ANOVA) to compare the hospitals in terms of patient numbers, occupancy rate and amount of medical waste and to determine any differences in these parameters over 3-month periods in each of the hospitals. The post hoc Bonferoni test was used to detect the groups that showed a difference after ANOVA. For each hospital, the paired t-test was used to identify any difference between the pre-pandemic and the pandemic periods in terms of patient numbers, occupancy rate and amount of medical waste. Statistical significance was set at P < 0.05.
Results
In the pre-pandemic period (April 2019–March 2020), the state hospital had the highest number of occupants with 48 187 inpatients, followed by the university hospital with 31 121 and the private hospital with 7 249. Correspondingly, the state hospital produced the greatest amount of waste (406 603 kg), with the university and private hospitals following. Table 1 shows changes in the amount of waste and number of patients seen in the 3 hospitals during the pre-pandemic and pandemic periods.
Table 2 presents the mean values for number of patients, amount of waste and occupancy rate for each hospital during the pre-pandemic and pandemic periods. Figure 1 reflects the amount of medical waste and the number of inpatients for the university, state and private hospitals during the same periods and shows the effect of the pandemic on these statistics. The number of patients was statistically significantly lower during the pandemic period in the university (P = 0.004) and state (P = 0.001) hospitals but significantly increased in the private hospital (P = 0.002). Although the university and state hospitals also demonstrated a decrease in their occupancy rates (P = 0.001 and P < 0.001; respectively), the changes were not statistically significant in the private hospital during the pandemic (P = 0.201). When the hospitals were cyclically compared in themselves, there was no statistically significant difference over the 3-month periods in the state hospital (P = 0.051). However, during the pandemic period, the university and private hospitals generated the highest amount of medical wastes in the 3-month period October–December (P = 0.020 and P = 0.028; respectively) (Table 2).
The mean values for patient numbers and amount of medical wastes during the pre-pandemic period were highest in the state hospital and lowest in the private hospital (P < 0.001 for both); in fact, all 3 hospitals were significantly different from each other (P < 0.001 for all comparisons). During the pre-pandemic period, the occupancy rate of the university hospital was lower than and significantly different from those of the state and university hospitals (P < 0.001 for both) (Table 2).
During the pandemic period, mean values for patient numbers at the private hospital were lower than and significantly different from those of the university and state hospitals (P < 0.001 for both). During the same period, mean values for occupancy rate at the private hospital were higher than and significantly different from those of the university and state hospitals (P < 0.001 for both). The mean values for amount of medical waste were also different, highest in the private hospital and lowest in the university hospital (P < 0.001 for all comparisons) (Table 2).
In the university hospital, in both the pre-pandemic and pandemic periods, as the patient numbers (P = 0.019 and P = 0.008 respectively) and occupancy rates (P = 0.024 and P = 0.002 respectively) increased, so did the amount of medical waste (Table 3). No significant relationship was found between number of patients and the amount of medical waste during the pre-pandemic and pandemic periods in the state hospital (P = 0.113 and P = 0.823 respectively). A significant relationship was found between occupancy rate and the amount of medical waste during the pre-pandemic and pandemic periods in the state hospital (P < 0.001 and P < 0.001 respectively). No significant correlation was found between number of patients and occupancy rate and the amount of medical waste in the private hospital during the pre-pandemic period (P = 0.550 and P = 0.424 respectively). We found that, in the private hospital during the pandemic period, as the number of patients and occupancy rate increased so did the amount of medical waste (P = 0.018 and P = 0.034 respectively) (Table 3).
Discussion and conclusion
In many countries, a national emergency was declared and restrictions on mobility and economic activities imposed due to the COVID-19 pandemic significantly affected waste production (10,11). Many companies and businesses switched to remote working. It has been reported that the infection rate decreased and mortality risk was reduced as a result of the social distancing measures taken (12–15). A significant reduction in hospital admissions was also observed during the lockdown periods (12,16). In comparison with the pre-pandemic period, we found that routine hospital admissions were restricted to prioritizing the care of critically-ill patients; the number of patients attending state and university hospitals for mere self-concern was noticeably lower, but the number attending the private hospital increased during this period. This may be interpreted as patients preferring to attend less-crowded private hospitals during the pandemic period. Additionally, patients preferring private hospitals tended to be older and opted for private hospitals due to the shorter waiting periods for test results, and the presence of a well-established population relying on private hospitals may also have impacted these findings (17).
Although 75–90% of waste generated in hospitals does not have any potential risk, the remaining 10–25% can be hazardous (2). It is known that better training of health care workers and standardization of waste management are key aspects of efficient waste management in health care facilities (18). Our study demonstrated that there was a significant increase in medical waste during the COVID-19 pandemic. It is normal for university hospitals to produce higher amounts of waste since they are research and training hospitals. Because this rate is also related to the technological infrastructure, the excessive rate of waste in the state hospital was attributed to the renewed high technological infrastructure. On reviewing the amount of medical waste in Turkey, it was found that the amount of medical waste was 0.91 kg/per person in 2016 and 1.10 kg/per person in 2019 (19,20). Compared with these figures, we observed that the amount of medical waste per person in the 3 hospitals we studied was quite high. Hence, these establishments need to review their waste management protocols as the source of medical wastes is predominantly the wards where inpatients are treated.
The available disposal strategies comprise the separation of wastes at the disposal site within the health care facilities and their transportation to a safe disposal site where the infectious medical waste is processed through incineration or autoclaving and the remainder is stored. There are disadvantages to both incineration and autoclave treatment methods. While incineration creates unwanted atmospheric emissions that cause negative health and environmental effects, autoclaving cannot be used to treat all kinds of waste, nor can it produce a universally accepted processed product for waste yards (18,21,22). Medical wastes collected in our setting are subjected to treatment through autoclaving and are disposed of afterwards by being buried at the storage site.
The best way to control the effect of medical wastes is to produce less, i.e. reduction at source, and one of the most effective ways to do this is to ensure that only infectious medical wastes are sent for special processing and treatment. Other hospital wastes such as packaging and domestic wastes need to be processed in a similar manner to that of municipality wastes (18). The COVID-19 virus is spread through sneezing, coughing, physical contact and contact with infected surfaces (23–26). The survival period for SARS-CoV-2 on objects/surfaces depends on the type of substrate and environmental conditions and ranges from a few hours to a couple of days. The long survival of SARS-CoV-2 raises the infection risk within a society (27,28). As a consequence of the panic caused by this information, all wastes from all wards where COVID-19 patients were treated were classed as medical wastes, consequently the amount of medical wastes increased. All wastes emanating from the clinic dedicated to the care of COVID-19 patients, including those from sterile dressing areas, have been processed as medical wastes. Yet, the bags and packages for the personal protective equipment and masks used in the wards could have been disposed of as domestic wastes like the masks and gloves used in the community for protective purposes. A similar approach could be seen in the state hospital, where medical waste production increased despite the reduced occupancy rates during the pandemic period. Several years ago, a report on “Hospital Waste Composition Research” from the Turkish Statistical Institute was presented by the General Directorate of Environmental Management. The total amount of solid wastes emanating from state and private hospitals and the distribution of the physical composition were investigated: the wastes were classified as medical wastes, domestic wastes and recyclable material. When the results of the survey were reviewed, it was seen that 0.09 kg per bed of recyclable waste was being produced daily in state hospitals, while in private hospitals this was 0.98 kg per bed per day (29). These data suggest that medical waste management is carried out more effectively in private hospitals. Our findings showed that, despite the decrease in patient numbers in both public hospitals, medical waste production increased. When changes in the amount of medical waste and the number of inpatients during the pre-pandemic and pandemic periods in the 3 hospitals were examined, it was seen that, even though patient numbers were much reduced in April 2020, the amount of medical waste slightly increased. This situation was associated with the fact that the state hospital cared for more COVID-19 patients than the university hospital did. The proportion of COVID-19 patients to the total number of patients in the hospitals during the pandemic had not been taken into consideration, therefore, this negative change in the state hospital can be attributed to the fact that more personal protective equipment was used during this time.
In addition, during the pandemic period, the university and private hospitals generated the greatest amount of medical wastes in the period October–December. This was when the second COVID-19 peak was experienced in our country and information on mutations was shared in the world for the first time. However, no significant observation could be made on how the amount of medical waste increased when patient numbers did not increase and occupancy rates did not change. In the private hospital in April–June, the occupancy rate was statistically significantly high, suggesting that patients who specifically preferred a private hospital considered the fact that this hospital had fewer patients and thus there was less risk of contact. Comparing the 3 hospitals, it was expected that the private hospital would have a high amount of medical waste since its occupancy rate was the highest. The low number of patients and the high occupancy rate of the private hospital are 2 outcomes in support of our argument that patients preferred the private hospital with fewer patients and a lower risk from contact (and also to get inpatient treatment).
It has been emphasized that many developing countries still lack the infrastructure to process their medical wastes as well as other infectious or hazardous wastes (30). As in the example of the COVID-19 pandemic, in the absence of an efficient waste management plan, wastes emanating from a health care facility may pose great problems. Despite the use of heat treatment, businesses may face more medical wastes than their capacities can process and treat. In such a situation, wastes need to be directed to storage sites to be stockpiled. It is recommended to create an area isolated from non-hazardous wastes for these wastes and to cover them up every day (23). The daily capacity of the medical waste disposal site in the geographical region covered by our study is 350 tons. Since medical wastes were not brought from other sites to this region for disposal, overcapacity was not encountered during the pandemic period.
To conclude, appropriate medical waste management is not only associated with the quality of services provided by the health care facilities but also reflects the welfare and level of consciousness of the institution and the country.
Funding: The authors declare the study received no funding.
Competing interests: The authors declare no conflict of interest.
References
- Chartier Y, Emmanuel J, Pieper U, Prüss A, Rushbrook P, Stringer R, et al. eds. Safe management of wastes from health-care activities, 2nd ed. Geneva: World Health Organization; 2014:p329 (http://apps.who.int/iris/bitstream/10665/85349/1/9789241548564_eng.pdf, accessed 20 March 2023).
- Askarian M, Heidarpoor P, Assadian O. A total quality management approach to healthcare waste management in Namazi Hospital, Iran. Waste Manag. 2010 Nov;30(11):2321-6. doi:10.1016/j.wasman.2010.06.020
- Kalina M, Ali F, Tilley E. “Everything continued as normal”: What happened to Africa’s wave of Covid-19 waste? Waste Manag. 2021 Feb 1;120:277–9. doi:10.1016/j.wasman.2020.11.051
- Tirkolaee EB, Abbasian P, Weber GW. Sustainable fuzzy multi-trip location-routing problem for medical waste management during the COVID-19 outbreak. Sci Total Environ. 2021 Feb 20;756:143607. doi:10.1016/j.scitotenv.2020.143607
- Singh E, Kumar A, Mishra R, Kumar S. Solid waste management during COVID-19 pandemic: recovery techniques and responses. Chemosphere. 2022 Feb;288(Pt 1):132451. doi:10.1016/j.chemosphere.2021.132451
- Dehal A, Vaidya AN, Kumar AR. Biomedical waste generation and management during COVID-19 pandemic in India: challenges and possible management strategies. Environ Sci Pollut Res Int. 2022 Feb;29(10):14830–45. doi:10.1007/s11356-021-16736-8
- Mekonnen B, Solomon N, Wondimu W. Healthcare waste status and handling practices during COVID-19 pandemic in Tepi General Hospital, Ethiopia. J Environ Public Health. 2021 Jan 30;2021:6614565. doi:10.1155/2021/6614565
- Rathnayake D, Clarke M, Jayasinghe VI. Health system performance and health system preparedness for the post-pandemic impact of COVID-19: a review. Int J Healthc Manag. 2021 Jan 2;14(1):250–4. doi:10.1080/20479700.2020.1836732
- Kalantary RR, Jamshidi A, Mofrad MMG, Jafari AJ, Heidari N, Fallahizadeh S, et al. Effect of COVID-19 pandemic on medical waste management: a case study. J Environ Health Sci Eng. 2021 Mar 18;19(1):831–6. doi:10.1007/s40201-021-00650-9
- Sarmento P, Motta M, Scott IJ, Pinheiro FL, de Castro Neto M. Impact of COVID-19 lockdown measures on waste production behavior in Lisbon. Waste Manag. 2022 Feb 1;138:189–98. doi:10.1016/j.wasman.2021.12.002
- Ali MA, Al-Khani AM, Sidahmed LA. Migrant health in Saudi Arabia during the COVID-19 pandemic. East Mediterr Health J. 2020 Aug 25;26(8):879–80. doi:10.26719/emhj.20.094
- Chowell G, Mizumoto K. The COVID-19 pandemic in the USA: what might we expect? Lancet. 2020 Apr 4;395(10230):1093–4. doi:10.1016/S0140-6736(20)30743-1
- Ali MY, Bhatti R. COVID-19 (Coronavirus) Pandemic: information sources channels for the public health awareness. Asia Pac J Public Health. 2020 May;32(4):168–9. doi:10.1177/1010539520927261
- Binns C, Low WY, Kyung LM. The COVID-19 pandemic: public health and epidemiology. Asia Pac J Public Health. 2020 May;32(4):140–4. doi:10.1177/1010539520929223
- Sayad B, Rahimi Z. Blood coagulation parameters in patients with severe COVID-19 from Kermanshah Province, Islamic Republic of Iran. East Mediterr Health J. 2020 Sep 24;26(9):999–1004. doi:10.26719/emhj.20.105
- Akıllı H, Bolankake N, Kuşçu ÜE, Haberal A, Ayhan A. Covid-19 pandemisi öncesi ve sonrasi uygulanan jinekolojik onkolojik cerrahilerin kisa dönem sonuçlarinin karşilaştirilması. [Comparison of the short-term results of gynecological oncological surgeries applied before and after the Covid-19 pandemic]. Sağlık ve Toplum [Health and Society]. 2021;31(1):54–9.
- He J, Hou XY, Toloo GS, FitzGerald G. Patients’ choice between public and private hospital emergency departments: a cross-sectional survey. Emerg Med Australas. 2017 Dec;29(6):635–42. doi:10.1111/1742-6723.12841
- Windfeld ES, Brooks MSL. Medical waste management – a review. J Environ Manage. 2015 Nov 1;163:98–108. doi:10.1016/j.jenvman.2015.08.013
- Sağlık kuruluşlari atik istatistikleri açıklandı [Health institutions waste statistics announced]. Ankara: Turkish Statistical Institute; 2016 (https://ohsad.org/tuik-2016-saglik-kuruluslari-atik-istatistikleri-aciklandi, accessed 20 March 2023).
- Eryılmaz H Demirarslan KO. 2012–2018 Yillari tibbi atiklarinin nüfus ileilişkilendirilmesi ve mevcut bertaraf yöntemlerininincelenmesi [Evaluation of 2012–2018 medical wastes with the population and current disposal methods]. ADYU Mühendislik Bilimleri Dergisi [ADYU J Engineering Sci]. 2020;13:89–103 (https://dergipark.org.tr/tr/download/article-file/1221371, accessed23March2023).
- Kudli LP, Jaishankar SS, Iyer RD. Biomedical waste management during the COVID-19 pandemic–Indian scenario. J Indian Assoc Environ Manage. 2021;41(1):41–53 (http://op.niscair.res.in/index.php/JIAEM/article/view/45615/465479117, accessed 20 March 2023).
- Ghasemi MK, Yusuff RM. Advantages and disadvantages of healthcare waste treatment and disposal alternatives: Malaysian scenario. Pol J Environ Stud. 2016;25(1):17–25. doi:10.15244/pjoes/59322
- Waste management during the COVID-19 pandemic, ISWA’s recommendations. Rotterdam: International Solid Waste Association; 2020 (https://www.humanitarianlibrary.org/sites/default/files/2020/07/ISWA_Waste_Management_During_COVID-19.pdf, accessed 20 March 2023).
- Duong DM, Le VT, Ha BTT. Controlling the COVID-19 pandemic in Vietnam: lessons from a limited resource country. Asia Pac J Public Health. 2020 May;32(4):161–2. doi:10.1177/1010539520927290
- Abid K, Bari YA, Younas M, Tahir Javaid S, Imran A. Progress of COVID-19 epidemic in Pakistan. Asia Pac J Public Health. 2020 May;32(4):154–6. doi:10.1177/1010539520927259
- Seyedin H, Moslehi S, Sakhaei F, Dowlati M. Developing a hospital preparedness checklist to assess the ability to respond to the COVID-19 pandemic. East Mediterr Health J. 2021 Feb 25;27(2):131–41. doi:10.26719/2021.27.2.131
- van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564–7. doi:10.1056/NEJMc2004973
- Basij-Rasikh S, Khalil M, Safi N. Early responses to COVID-19 in Afgankara:hanistan. East Mediterr Health J. 2020 Dec 9;26(12):1442–5. doi:10.26719/emhj.20.137
- Güvenli tibbi atik yönetimi [Safe Medical Waste Management]. Ankara: Ministry of Environment and Urbanization General Directorate of Environmental Management; 2013 (https://webdosya.csb.gov.tr/db/cygm/editordosya/guvenliatikkilavuz.pdf, accessed 20 March 2023).
- Sawalem M, Selic E, Herbell JD. Hospital waste management in Libya: a case study. Waste Manag. 2009 Apr;29(4):1370–5. doi:10.1016/j.wasman.2008.08.028
Observational study and literature review of the use of camel urine for treatment of cancer patients
Ali Al Zahrani1, Ali Alfakeeh1 , Waleed Alghareeb1, Hatoon Bakhribah1, Bassam Basulaiman1, Abdullah Alsuhail2 and Abdullah Alsharm1
1Medical Oncology Department, Comprehensive Cancer Center, King Fahad Medical City, Riyadh, Saudi Arabia (Correspondence: A. Al Zahrani:
Abstract
Background: Complementary and alternative medicine is widely used in Saudi Arabia. One of the commonly used methods is camel urine alone or mixed with camel milk, which is supported by vague religious beliefs.
Aims: To observe and follow up our cancer patients who insisted upon using camel urine, and to devise some clinically relevant recommendations.
Methods: We observed 20 cancer patients (15 male, 5 female) from September 2020 to January 2022 who insisted upon using camel urine. We documented the demographics of each patient, the method of administering camel urine, the reasons for refusing conventional treatment, the period of follow-up, and the outcome and side effects.
Results: All the patients had radiological investigations before and after finishing treatment with camel urine. All patients used a combination of camel urine and milk, and treatment ranged from a few days up to 6 months. The average amount of urine/milk consumed was 60 ml/day. No clinical benefit was observed and 2 patients developed brucellosis. Eleven patients changed their mind and accepted conventional antineoplastic treatment but 7 were too weak to receive further treatment and died from their disease.
Conclusion: Camel urine had no clinical benefits in cancer patients, and may even have caused zoonotic infection. The promotion of camel urine as a traditional medicine should be stopped because there is no scientific evidence to support it.
Keywords: camel urine, camel milk, cancer, religious treatment, complementary and alternative medicine
Citation: Al Zahrani A, Alfakeeh A, Alghareeb W, Bakhribah H, Basulaiman B, Alsuhail A, et al. Observational study and literature review of the use of camel urine for treatment of cancer patients. East Mediterr Health J. 2023;29(8):xxx-xxx https://doi.org/10.26719/emhj.23.050
Received: 20/06/22; accepted: 15/12/23
Copyright: © Authors; licensee World Health Organization. EMHJ is an open access journal. All papers published in EMHJ are available under the Creative Commons Attribution Non-Commercial ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Introduction
Complementary and alternative medicine is widely used in Saudi Arabia as part of traditional health care and practice, and it is preferred by some cancer patients because they think it is safe and natural compared with chemotherapy and radiotherapy (1). One of the commonly used forms of complementary and alternative medicine is camel urine alone or in combination with camel milk. This practice is supported by a story from Islamic tradition (2, 3).
We have noticed through our daily practice that many of our patients use this type of therapy on the recommendation of others and sometimes without informing their treating physicians. Healthcare providers do not recommend this treatment, especially for those who are still in active conventional treatment, but many of our patients still insist on trying it.
Some preclinical data have shown some anticancer properties of camel urine but it has not been investigated clinically (5–7). Other cultures use urine from cows or other animals, and some even use human urine (16, 20).
An outbreak of MERS-CoV occurred in Saudi Arabia in 2012 and camels were suspected as a possible original source of the virus. This led the World Health Organization (WHO) to recommended that any consumption of raw or undercooked animal products, including milk, urine, and meat, should be avoided (13, 14).
In this study, we observed and followed up our cancer patients who insisted upon using camel urine to establish exactly how they administered the urine and whether there were any clinical benefits or harm. This is possibly the first clinical report on the effect and side effects of camel urine in human cancer patients who insisted upon using camel urine instead of established antineoplastic agents.
Methods
We estimated that a sample size of 66 patients would be needed, based on the local prevalence of camel milk/urine consumption of ~24%. The sample size was calculated using the statistical formula n = Z2 P(1-P)/d2, where Z was the statistic corresponding to level of confidence, P was the expected prevalence, and d was the precision. We included patients who were supposed to start chemotherapy between September 2020 and January 2022 but refused to receive it. Instead, the patients insisted upon using camel urine, despite receiving counselling that this type of treatment is unproven and any delay in their treatment might be harmful. The patients gave signed informed consent and baseline computed tomography and laboratory investigations were conducted. All patients were made fully aware that they could change their mind any time and return to their antineoplastic treatment. The study was approved by the local ethical committee of King Fahad Medical City (21-339).
All patients in the study used camel urine mixed with milk and nothing else, because the usual advice is not to mix it with other medication. We only observed the first 20 patients because we noticed that they all had disease progression and it would have been unethical to wait until we recruited the required 66 patients. We documented the demographics of each patient, urine use, reasons for refusing conventional treatment, duration of follow-up, treatment outcome, and side effects. The follow-up included history, physical examination, laboratory investigations, tumour markers, and radiological investigations. All patients underwent a radiological or biochemical investigation or both before and after stopping consumption of camel urine. We decided to report the first 20 patients because the results might contribute to changing the current beliefs about consuming camel urine for cancer treatment.
Results
We interviewed 20 patients (15 male, 5 female) with different types of cancers at different stages. All patients underwent radiological investigations, mainly computed tomography, before and after finishing consumption of camel urine. Magnetic resonance imaging was also used for patients with brain tumours, rectal cancer, and soft tissue and bone sarcoma. We measured carcinoembryonic antigen for colon and oesophageal cancer, a-fetoprotein for hepatocellular carcinoma, and carbohydrate antigen 19-9 for hepatobiliary cancer. All patients used a mixture of camel urine and milk (Table 1). The duration of consumption ranged from 3 days to 6 months, and the average amount was ~60 ml/day (volumes were estimated because most patients did not use a measured container).
Twelve patients developed metastases and the others had locally advanced or recurrent cancer. None of the patients who continued to use camel urine showed any clinical benefit. There was no improvement in tumour-related symptoms, and patients showed radiological progression and an increase in tumour markers. Two patients developed brucellosis: 1 was successfully treated with antibiotics and returned to chemotherapy; and the other developed severe respiratory failure with lung infiltration with no obvious cause. No autopsy was done on the latter patient because this is not a routine procedure in our health system (except after a court request); however, the clinical features were similar to those of MERS-CoV pneumonia. The rest of the patients had disease progression. Eleven patients changed their mind and accepted conventional antineoplastic treatment but 7 were too weak to receive any further treatment and died and 2 are still being followed up (Table 1).
All of the patients believed that camel urine was a religious treatment and the advice came mainly from the surrounding community. One patient had followed specific instructions published on Twitter promoting a schedule for the use of camel urine for cancer treatment (Faten Khorshid, The quantities that a person can consume of camel milk and urine and the method of changing the taste. Twitter post, 14 January 2022; https://twitter.com/FatenProf/status/1536550514850152449?t=aC9oTmwwvt7_PPblEUKh9Q&s=08).
Discussion and literature review
The main support for the use of camel urine mixed with camel milk came from a story from Islamic tradition. Some people from the Orayna tribe that used to live in the central part of the Arabian Peninsula came to The Prophet in Medina pretending to be Muslims. They could not tolerate city life and became unwell and had abdominal distention. The Prophet asked them to go outside the city to a herd of camels, and told them to drink their milk and urine as a medicine because it was a common practice for those living in the desert. After doing so, they became healthy. (2, 3). There is a disagreement among Islamic scholars about whether camel urine is unclean. The Maliki and Hanbali schools of jurisprudence hold that the urine of animals whose meat can be consumed is considered to be clean (4). In contrast, the Shafii and Hanafi schools consider camel urine to be unclean. The Prophet’s recommendation to use camel urine was only in an emergency, for a specific illness, and because no clean medicine was available (4).
There were some preclinical studies that claimed that camel urine had several therapeutic benefits, such as antimicrobial, anti-inflammatory, and anticancer activities, and even some beneficial cardiovascular effects. One study showed that camel urine inhibited growth of hepatocellular carcinoma (HEPG2), colon carcinoma (HCT 116), human glioma (U251), lung cancer, and leukaemia cells (5). Further studies showed that camel urine blocked cytochrome P450 1a1, a cancer-causing gene, and inhibited inflammatory angiogenesis, which can supply nutrition to tumours (6, 7). Successful anticancer agents kill cancer cells or prevent their proliferation without negatively affecting normal cell growth or disrupting the immune system (8). These features were claimed to have been seen in another study of camel urine (9). A variety of tests conducted in vivo and in vitro demonstrated the ability of camel urine to reduce or prevent the metastatic potential of breast cancer cells (10).
The possible mechanism of action of camel urine was through apoptosis and suppression of cancer cells (10). The possible anticancer properties of camel urine have been vaguely demonstrated using gas chromatography/mass spectrometry and inductively coupled plasma mass spectrometry (11). That study showed higher concentrations of metabolites such as canavanine in camel urine than in other mammals’ urine. Canavanine is a byproduct of amino acid and urea metabolism, and according to that study, it was potent in combating tumour cells. Camel urine has a low amount of urea and ammonia that may decrease its unpleasant odour and toxicity when consumed by humans; however, there is a large amount of creatine and creatinine, which are toxic to humans (12). The mineral content of camel urine is 10-fold greater than that of human urine, and it is usually alkaline with pH > 7.8, while human urine is usually acidic (12).
MERS was first discovered in 2012 in Saudi Arabia and Jordan, and camels were identified as the reservoir host. This disease spreads through close contact, directly or indirectly, with camels and can also spread among infected humans (13). The causative agent MERS-CoV is a zoonotic virus that spreads from animals to humans. The disease was found to be harmless to camels but infection in humans can be severe, with 35% of all human cases resulting in death. To date, 27 countries have been affected by MERS. The method of transmission of MERS-CoV from animals to humans remains unknown. Therefore, WHO recommends that any activities related to camels must be avoided, such as close contact with dromedary camels, drinking raw camel milk or urine, or eating meat that has not been properly cooked (14).
None of the basic scientific studies about the use of camel urine in cancer patients present sufficient evidence for its use in modern medicine. Most studies are still in their early stages, using in vivo and in vitro studies on animal cells, and do not involve actual patients. There are major concerns about the correlation between preclinical and clinical data. They are not always strongly correlated and the activity of some anticancer agents in preclinical studies has not been translated into clinical benefit (26). An analysis was carried out by the National Cancer Institute Developmental Therapeutics Program on the activity of 39 compounds tested preclinically in vivo and in vitro assays, and comparing the results with corresponding data from Phase 2 clinical trials (15). The results indicated the weak correlation of preclinical models with clinical results and confirmed the need for clinical trials before approving any therapeutic methods.
Urine from other species is used worldwide. In some areas, consumption of human urine is practiced and it became popular after publication in the early 20th century of Water of life by the British naturopath John W. Armstrong (16). Human urine mainly consists of water (> 95%). The remaining constituents are urea 9.3 g/l, chloride 1.87 g/l, sodium 1.17 g/l, potassium 0.750 g/l, creatinine 0.670 g/l, and other dissolved ions and inorganic and organic compounds (17). Although it is widely used, there is no scientific evidence for therapeutic use in humans and harmful effects have been reported (18). Urine from goats, sheep, elephants, donkeys, and other animals has also been used for treatment by different cultures (19). In India, cow urine was studied in preclinical trials (20). It was found to contain nitrogen, sulfur, phosphate, sodium, manganese, iron, silicon, chlorine, magnesium, maleic, citric, and tartaric acids, calcium salts, vitamins A–E, minerals, lactose, enzymes, creatinine, and hormones. The authors claimed that the ingredients in cow urine were similar to those in the human body, which is why they thought it could maintain the balance of these substances in humans if ingested, and may even cure some diseases (20). The United States of America has granted patents (Nos. 6,896,907 and 6,410,059) for the medicinal properties of cow urine, with particular mention of its bioenhancer, antibiotic, antifungal, and anticancer properties. Cow urine increased the potency of paclitaxel against MCF-7, a human breast cancer cell line, in in vitro assays (US Patent No. 6,410,059) (21). The composition of cow urine is similar to that of other types of urine; it is mainly water (95%) and urea (2.5%), with the remainder made up of minerals, salts, hormones, and enzymes (2.5%) (22). According to a study from India, there was some evidence that cow urine had antioxidant properties and the ability to repair damaged DNA, and was therefore, an effective anticancer therapy (23). In a study of regression of induced papilloma in Swiss albino mice, cow urine reduced the incidence of papilloma, tumour yield, and tumour burden (24). In a study of different types of cancer, cow urine caused a decrease in the severity of clinical symptoms (pain, inflammation, burning sensation, difficulty swallowing, and irritation) from day 1 to day 8 (25).
None of our cancer patients showed any clinical benefit from the use of camel urine and this was documented as radiological progression, increase in tumour markers, or both. Two of the patients developed brucellosis, and 1 was successfully treated with antibiotics and returned to chemotherapy. There was 1 patient with possible MERS who died undiagnosed. This was not confirmed by laboratory testing but diagnosis of MERS was supported by the clinical and radiological features.
We know that > 95% of urine is composed of water and the remaining 5% is trace elements. The preclinical studies on camel urine were performed on these trace elements. Most of those studies suggested that the effectiveness of camel urine was because of the presence of trace elements that had anticancer activity. However, for the trace elements to have a clinical effect would require consumption of a huge volume of urine, and even then, the trace elements would be diluted. We also know that not all preclinical trials can be translated into clinically beneficial outcomes. It has been reported that only 5 in 5000 medications will progress successfully from preclinical to clinical studies, and only 1 of the 5 will be clinically beneficial, and even that may not be better than existing medication (26).
When we look to the story that happened 1440 years ago, which supported the use of camel urine, it definitely does not describe cancer patients because the patients appeared to have symptoms of a communicable disease that developed over a short period of time. Many Islamic scholars believe that the advice to drink camel urine and milk was specific to those particular people because they were used to the practice, but it was not common among others.
Conclusion
To the best of our knowledge, this is the first study to explore the clinical benefit of camel urine for treatment of cancer patients. All our cancer patients who insisted upon using camel urine did not have any significant clinical benefits and we do not recommend its use. Also, some of our patients presented with serious side effects. Two patients developed brucellosis because of the advice to use raw unboiled camel urine/milk to obtain the maximum benefit. We reported the results from a smaller number of patients than initially planned because we considered it important that promotion of this type of treatment as a traditional medicine should be stopped, because there is no evidence to support it scientifically.
Data availability: All relevant data are available and could be provided upon request to the corresponding author.
Conflict of interest: No conflicts of interest are declared.
Funding: No funding was received for this study.
References
- Alrowais NA, Alyousefi NA. The prevalence extent of complementary and alternative medicine (CAM) use among Saudis. Saudi Pharm J. 2017 Mar;25(3):306–18. https://doi.org/10.1016/j.jsps.2016.09.009 PMID:28344484
- al-Bukhari M. Sahih al-Bukhari volume 8, book 82, hadith 794. Damascus: Dar Ibn Katheer; 2002.
- al-Nesaburi M. Sahih Muslim, book 28, hadith 14. Cairo: Dar Ihea Alkutob Alarabiah; 1955.
- Mohammad I. Alshangeti Sharh Zad Almostagnae, book 23, page 9. Riyadh: General Presidency of Islamic Research and Ifta; 2007.
- Khorshid, F. Preclinical evaluation of PM 701 in experimental animals. Int J Pharmacol. 2008;4(6):443–51. https://doi.org/10.3923/ijp.2008.443.451
- Alhaider AA, El Gendy MAM, Korashy HM, El-Kadi AOS. Camel urine inhibits the cytochrome P450 1a1 gene expression through an AhR-dependent mechanism in Hepa 1c1c7 cell line. J Ethnopharmacol. 2011 Jan 7;133(1):184–90. https://doi.org/10.1016/j.jep.2010.09.012 PMID:20883769
- Alhaider AA, Gader AGM, Almeshal N, Saraswati S. Camel urine inhibits inflammatory angiogenesis in murine sponge implant angiogenesis model. Biomed Aging Pathol. 2014 Jan–Mar;4(1):9–16. https://doi.org/10.1016/j.biomag.2013.10.003
- Ali A, Baby B,Vijayan, R. From desert to medicine: a review of camel genomics and therapeutic products. Front Genet. 2019 Feb 19;10(17):1–20. https://doi.org/10.3389/fgene.2019.00017 PMID:30838017
- Al-Yousef N, Gaafar A, Al-Otaibi B, Al-Jammaz I, Al-Hussein K, Aboussekhram A. Camel urine components display anti-cancer properties in vitro. J Ethnopharmacol. 2012 Oct 11;143(3):819–25. https://doi.org/10.1016/j.jep.2012.07.042 PMID:22922085
10) Romli F, Abu N, Khorshid FA, Syed Najmuddin SUF, Keong YS, Mohamad NE, et al. The growth inhibitory potential and antimetastatic effect of camel urine on breast cancer cells in vitro and in vivo. Integr Cancer Ther. 2017 Dec;16(4):540–55. https://doi.org/10.1177/1534735416656051 PMID:27338742
11) Ahamad SR, Alhaider AQ, Raish M, Shakeel F. Metabolomic and elemental analysis of camel and bovine urine by GC–MS and ICP–MS. Saudi J Biol Sci. 2017 Jan 24(1):23–9. https://doi.org/10.1016/j.sjbs.2015.09.001 PMID:28053567
12) Read BE. (1925). Chemical constituents of camel’s urine. J Biol Chem. 64:615–7.
13) Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012 Nov 8;367(19):1814–20. https://doi.org/10.1056/NEJMoa1211721 PMID:23075143 Erratum in: N Engl J Med. 2013 Jul 25;369(4):394.
14) Middle East respiratory syndrome coronavirus (MERS-CoV) [website]. Geneva: World Health Organization; 2019 (https://www.who.int/health-topics/middle-east-respiratory-syndrome-coronavirus-mers#tab=tab_1, accessed 15 February 2023).
15) Johnson J, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001 May 18;84:1424–31. https://doi.org/10.1054/bjoc.2001.1796 PMID:11355958
16) Armstrong JW. The water of life: a treatise on urine therapy. Random House; 2011.
17) Putnam DF. Composition and concentrative properties of human urine. NASA; 1971.
18) Ogunshe AAO, Fawole AO, Ajayi VA. Microbial evaluation and public health implications of urine as alternative therapy in clinical pediatric cases: health implication of urine therapy. Pan Afr Med J. 2010 May 25;5:12. https://doi.org/10.4314/pamj.v5i1.56188 PMID:21293739
19) Thakur AN. Therapeutic use of urine in early Indian medicine. Indian J History Sci. 2004;39(4):415–27.
20) Pathak ML, Kumar A. Cow praising and importance of Panchyagavya as medicine. Sachitra Ayurveda 2003;5:569.
21) Randhawa GK. Cow urine distillate as bioenhancer. J Ayurveda Integr Med 2010 Oct;1:240–1. https://doi.org/10.4103/0975-9476.74089 PMID:21731367
22) Cow urine content [website] (http://samvadsetu-gaumata.blogspot.in/2010/12/cow-urine-content.html, accessed 15 February 2023).
23) Kumar A, Kumar P, Singh LK, Agrawal OK. Pathogenic effects of free radicals and their prevention through cowpathy. Indian Cow 2009;6(21):4–8.
24) Raja W, Agrawal RC. Chemopreventive potential of cow urine against 7, 12 dimethyl benz(a) anthracene-induced skin papillomasgenesis in mice. Acad J Cancer Res. 2010;3(1):7–10.
25) Jain NK, Gupta VB, Garg R, Silawat N. Efficacy of cow urine therapy on various cancer patients in Mandsaur District, India – a survey. Int J Green Pharm. 2010;4:29–35.
26) Kraljevic S, Stambrook PJ, Pavelic K. Accelerating drug discovery. EMBO Rep. 2004;5(9):837–42. https://doi.org/10.1038/sj.embor.7400236
27) https://twitter.com/FatenProf/status/1536550514850152449?t=aC9oTmwwvt7_PPblEUKh9Q&s=08
Table 1. Characteristics and outcome of 20 patients who insisted upon using camel urine
|
Diagnosis |
Gender |
Age, yr |
Approximate daily amount of urine/milk |
Duration of consumption |
Evaluation method |
Side effects |
Outcome |
|
Metastatic cholangiocarcinoma |
Male |
67 |
20 ml/milk |
3 months |
CT, CA 19-9 |
Brucellosis |
Progression and death |
|
Metastatic colon cancer |
Male |
62 |
20 ml/milk |
4 months |
CT, CEA |
Brucellosis |
Progression returned to chemotherapy Alive |
|
Metastatic colon cancer |
Female |
54 |
20 ml/milk |
2 months |
CT, CEA |
Nausea and bad smell |
Progression returned to chemotherapy Alive |
|
Hepatocellular carcinoma |
Male |
62 |
20 ml/milk |
1 month |
CT, AFP |
Tolerable |
Progression and death |
|
Metastatic nasopharyngeal carcinoma |
Male |
66 |
15 ml/milk |
3 months |
CT |
Tolerable |
Progression and death |
|
Metastatic pancreatic cancer |
Female |
70 |
20 ml/milk |
2 months |
CT |
Bad smell and vomiting |
Progression and death |
|
Locally advanced rectal cancer |
Male |
62 |
50 ml/milk |
6 months |
CT, MRI, CEA |
Tolerable |
Local recurrence Alive |
|
Metastatic gallbladder carcinoma |
Male |
65 |
20 ml/milk |
4 months |
CT, CA 19-9 |
Tolerable |
Progression returned to chemotherapy Alive |
|
Metastatic small bowel clear cell sarcoma
|
Female |
38 |
20 ml/milk for 1 month then increased gradually to 300 ml for 4 months |
5 months |
CT |
Tolerable |
Progression, Alive |
|
Bladder cancer |
Male |
40 |
30 ml |
3 months |
CT |
Tolerable |
Disease progression Alive |
|
Recurrent glioblastoma |
Female |
47 |
50 ml |
2 months |
CT, MRI |
Tolerable |
Disease progression Alive |
|
Oesophageal adenocarcinoma (recurrent) |
Male |
47 |
30 ml |
1 month |
CT, CEA |
Tolerable |
Disease progression Returned to chemotherapy Alive |
|
Metastatic squamous cell carcinoma of the scalp |
Female |
57 |
50 ml |
3 months |
CT |
Tolerable |
Disease progression Returned to chemotherapy Died |
|
Hepatocellular carcinoma |
Male |
79 |
30 ml |
4 months |
CT, AFP |
Tolerable |
Disease progression Died |
|
Metastatic colon cancer |
Male |
63 |
40 ml |
3 months |
CT, CEA |
Nausea, vomiting |
Disease progression Return to chemotherapy Alive |
|
Metastatic Ewing sarcoma |
Male |
27 |
200 ml
|
2 weeks |
CT/MRI |
Tolerable |
Disease progression Returned to chemotherapy Alive |
|
Non-small cell lung cancer, squamous cell carcinoma |
Male |
70 |
50 ml |
4 months |
CT |
Tolerable |
Disease progression Returned to chemotherapy Alive |
|
Non-small cell lung cancer, adenocarcinoma |
Male |
68 |
100 ml |
3 days |
CT |
? MERS-Cov |
Hypoxia and respiratory failure, bilateral lung infiltrate Died |
|
Metastatic pancreatic cancer |
Male |
67 |
60 ml |
4 months |
CT, CA 19-9 |
Tolerable |
Disease progression Returned to chemotherapy Alive |
|
Locally advanced squamous cell carcinoma of the tongue |
Male |
60 |
60 ml |
4 months |
CT |
Tolerable |
Disease progression Returned to chemotherapy Alive |
AFP = a-fetoprotein; CA 19-9 = carbohydrate antigen 19-9; CEA = carcinoembryonic antigen; CT = computer tomography;
MRI = magnetic resonance imaging.
Quality of life of patients with thalassaemia living in the West Bank and Gaza
Pamela Kohlbry,1 Bashar al-Karmi2 and Robert Yamashita3
1School of Nursing, California State University San Marcos, San Marcos, CA, United States of America. 2Thalassemia Patients Friends Society, Ramalla, West Bank, Palestine. 3California State Universit y San Marcos, San Marcos, CA, United States of America. Correspondence to Pamela Kohlbry:
Abstract
Background: In countries with low resources, the health and quality of life of people with thalassaemia can be severely affected.
Aims: This study examined the health-related quality of life of people with thalassaemia in the West Bank and Gaza, Palestine.
Methods: This was a cross-sectional study of a convenience sample of 104 patients (71 adults and 33 children) with thalassaemia and their families conducted in 2015 in the West Bank and Gaza. Participants were surveyed using the 36-item Short Form Health Survey, version 2 (SF36v2), Pediatric Quality of Life InventoryTM (PedsQL) and PedsQL Family Impact Module to assess their quality of life. With the SF36v2, we used normed-based scoring. For the PedsQL and Family Impact Module, we used the 0–100 scoring. Scores are reported as means and standard deviations and P < 0.05 considered statistically significant.
Results: Scores were low across all domains indicating poor quality of life. For bodily pain in the SF36v2, a significant difference was seen between the West Bank and Gaza. No significant differences were found between males and females. Data from the PedsQL showed no significant differences between the West Bank and Gaza. With the Family Impact Module, the summary score was higher in adults than in the paediatric patients. Compared with other countries, thalassaemia patients in Palestine generally had lower quality of life scores in most domains.
Conclusion: The lack of access to health care and blood transfusions and geopolitical challenges may explain the low quality of life scores of patients with thalassaemia in Palestine.
Keywords: thalassaemia; quality of life; cross-sectional study; West Bank and Gaza.
Citation: Kohlbry P; al-Karmi B; Yamashita R. Quality of life of patients with thalassaemia living in the West Bank and Gaza. East Mediterr Health J. 2023;29(6):xxx–xxx. https://doi.org/10.26719/emhj.23.045
Received: 20/04/22 ; accepted: 21/11/22
Copyright: © Authors; licensee World Health Organization. EMHJ is an open access journal. All papers published in EMHJ are available under the Creative Commons Attribution Non-Commercial ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Introduction
Worldwide, genetic haemoglobinopathies such as thalassaemia are considered serious health burdens. In the United States of America, thalassaemia is rare and considered a disease of migration and it is managed as a chronic illness (1,2). However, in countries with fewer resources, the health burden of thalassaemia patients, which will also be a family burden, affects health-related quality of life (3,4). Thalassaemia is common in the Mediterranean, Middle East and sub-Saharan Africa (5). The prevalence of thalassaemia in Palestine which includes the Occupied West Bank and Gaza, is high; about 4% of the population are carriers (4). The political conflict and often violent conditions in Palestine complicate medical care of patients and their families who need to make regular clinic visits. Blood supply for transfusions is limited and chelation medication treatments are expensive or unavailable. Furthermore, continuity of care is lacking, social and psychological support services are limited, and there are few health care workers with specialized training in the management of thalassaemia. The World Health Organization (WHO) lists Palestine as having the second-lowest health expenditure as a percentage of the Gross National Product out of 18 Middle Eastern countries (6).
The quality of life and outcomes of people with thalassaemia vary depending on clinical care and other circumstances. In Palestine, thalassaemia patient-reported health outcomes are poorly understood, and clinical research data are limited (7–10).
Research that measures quality of life can help identify the effect of chronic illness on patients’ lives and allows comparison of different groups (11).
This study aimed to identify the quality of life of people with thalassaemia in the West Bank and Gaza. In 2009, we conducted a pilot study on the quality of life of patients with thalassaemia in Palestine (12). The study was designed to answer the question: could behavioural and social science research data be systematically collected in contested regions, classified by WHO as a low-income country? Our study in 2015 is a follow-up to that pilot. During the time of this study, regional conflict was a challenge to conducting this research (13,14). This study also compares Palestinian data with other populations such as Turkey, Thailand, Italy, Greece and Middle Eastern countries.
Methods
Sample, instruments and data collection
Both the 2009 and 2015 studies were cross-sectional studies using a convenience sample recruited from regional clinics in Palestine. In 2009, only adults were part of the sample. In 2015 we expanded the sample to include children and their guardians. Data were collected on demographic characteristics of the sample. In addition, three standardized quality of life tools were used: the 36-item Short Form Health Survey, version 2 (SF36v2), Pediatric Quality of Life InventoryTM (PedsQL) and PedsQL Family Impact Module (FIM).
Patients with thalassaemia and guardians of patients with thalassaemia who attended a transfusion clinic were approached about the study. If they were interested in joining the study, they were provided with information and an informed consent form to sign. If they chose to participate and signed the consent form, they were given the surveys to complete.
The instruments used in the study measured a patient’s quality of life and the impact the patient had on their family. The widely used SF36v2 instrument measures quality of life for young adults (ages ≥ 16 years) and older patients (15). The 36-item questionnaire measures eight dimensions of general health-related quality of life: physical functioning, role physical (limitation because of physical health problems), bodily pain, general health, vitality, social functioning, role emotional (limitations due to emotional problems), and general mental health. In addition, two summary scores assess physical and mental dimensions of health and well-being: physical component summary score and the mental component summary score. A high score indicates a more favourable health state. The instrument has been used extensively in clinical trials and academic studies, across disease areas, including thalassaemia. Its validity and reliability to measure health-related functional status and well-being have been established (15). It has a high level of consistency across countries and the Arabic translation has been validated (16).
The PedsQL 4.0 generic core scales contain 23 items grouped into four scales: physical functioning, emotional functioning, social functioning and school functioning. It also includes composite scales for a total scale score (23 items) and a psychosocial health summary score which is the sum of emotional, social, and study/work functioning. A high score indicates a more favourable health state (17–19). All paediatric patients and their caregivers were surveyed using the PedsQL.
The FIM survey has 36 items and measures the effect of chronic paediatric health conditions on parents and the family. The survey measure six scales of parent self-reported functioning: physical functioning, emotional functioning, social functioning, cognitive functioning, communication and worry. It also measures two scales for parent-reported family functioning: daily activities and family relationships (20).
In 2009 we used the SF36v2. In 2015, we assessed paediatric health-related quality of life using the PedsQL. In addition, because of the chronic nature of transfusion-dependent thalassaemia, we used the PedsQL FIM to assess the impact the disease had on caregivers.
Statistical analysis
The SF36v2 manual provides a standardized approach for statistically analysing the data. It allows for a systematic approach for converting the individual data points into a meaningful component for a scale. The initial step converts the score into a 0–100 range with 0 (worst health) to 100 (best health) results. The SF36v2 designers recognized that the component nature of the scales and what they intended to measure were widely variable; the range between top and bottom scores varied substantially across health domains. Without understanding this, the data could be easily misinterpreted. Therefore, in order to simplify interpretation and make direct comparisons, a norm-based scoring approach can be used with the SF36v2, where each scale has the same average (50) and same standard deviation (SD) (11). This approach makes the health impact of a domain norm-based scoring score clearer and can more accurately reflect the impact of the disease. An individual score of < 45 or a group score of < 47 indicates that quality of life is severely affected. Norm-based scoring requires the assessment of a general population, and because SF36v2 was developed in the United States (US), it uses the US population as its norm.
The PedsQL and the FIM were also scored using the 0–100 scale. For comparisons across studies, the P values were used for all instruments. P less than 0.05 was considered statistically significant.
Ethics
The study was approved by the Institutional Review Board Committee of the California State University San Marcos and the ethics review and administrative authority of the clinics where the participants were drawn. Support to conduct the study and obtain participants was provided by the Thalassemia Patients Friends Society. The Thalassemia Patient’s Friends Society is a non-profit Palestinian nongovernmental organization working to build patient capacity to manage health, economic and social aspects to improve their quality of life (21,22). All patients who visited clinics at the time of the study were invited to participate voluntarily. Patients were excluded only if they chose not to participate.
Results
Characteristics of the sample
At the time of the 2015 study, there were 750 people with thalassaemia and their families in the West Bank and Gaza. Of these people, 104 participated in the study: 65 from the West Bank and 39 from Gaza. Our sample size was adequate compared with other published studies on thalassaemia. Seventy-one participants completed the SF36v2, and 33 paediatric participants completed the PedsQL (Table 1). The participants were distributed fairly equally by sex: 51 males and 52 females overall (data on sex were missing for one person). The age range was < 10 years to ≥ 30 years; most were between 15 and 24 years. Of the 33 participants who completed the PedsQL, 19 were from Gaza and 14 from the West Bank.
SF36v2 results
In the 2015, 71 participants completed the SF36v2 (Table 2). Most participants were from the West Bank, 46 compared with 25 from Gaza. SF36v2 scores were poor for physical functioning and mental health. The physical health component scores showed a statistically significant difference between Gaza (40.53) and the West Bank (39.49). However, the mental health component scores showed no statistical differences, but Gaza patient scores were lower than the West Bank. Gaza scored lower in most domains than the West Bank with the lowest in the mental health component score (31.45). Male and female scores were low in most areas with females scoring lower for bodily pain (34.52), social function (33.67), mental health (33.29) and mental health component score (32.46). The only significant difference in scores in the various domains was between Gaza and West Bank for bodily pain; no significant difference was seen between the sexes. Male participants in the West Bank scored significantly lower for bodily pain compared with male and female participants in Gaza. In the analysis by location and sex, the lowest scores were in the mental component summary.
PedsQL results
Of the 33 participants who completed the PedsQL, 19 were from Gaza and 14 from the West Bank (Table 3). Scores in Gaza were consistently lower than the West Bank scores across all domains. However, these differences were not statistically significant. Most (61%) of the participants were female. In the child self-report, females generally scored lower than males, except for school functioning. The lowest score for females was for physical functioning (54.06). The differences between males and females were not significant. The age range was < 10 (27%), 10–14 years (36%) and ≥ 15 years (36%). The oldest age group scored the lowest for all domains. Parents scored children 10–14 years the highest, except for school functioning where parents scored both younger and older age groups higher (Table 3).
FIM results
In the Family Impact Module scoring, the 104 participants who completed the survey scored lowest in physical functioning and emotional functioning (Table 4). Overall, males had lower scores than females. Parents perceived that younger children were harder to understand when they communicated on their health care. However, as the children got older, the scores generally increased indicating that overall the families seem to have adapted to thalassaemia health care needs.
Discussion
PedsQL comparison
In comparison with studies using the PedsQL in Turkey, in Middle-Eastern patients in Italy and in Thailand, the Palestinian scores were consistently lower across all domains (Table 5) (23–25). The PedsQL scores were significantly higher in Turkey and Thailand in the domains of emotional functioning, social functioning, psychosocial health summary score and overall functioning (23,24). Scores in Thailand were also significantly higher for physical functioning (24). The scores in Italy were not significantly different from our Palestinian scores (25). These statistically significant lower findings may reflect environmental challenges related to lack of resources. Data collection for our study was during a period of substantial upheaval and conflict in Palestine.
FIM comparison
The FIM results demonstrate that Palestinian parents are affected similarly compared with studies in other areas (Table 6). Palestinian parents appeared to have poorer quality of life on the parental quality of life measures compared with other studies, but the differences were not statistically significant. With the family quality of life, Palestinian families appear to be doing better in the overall domain, although again no significant differences were found except in the domain of daily activities (26). The effect on the family of having a child with thalassaemia was similar across studies (Table 6).
SF36v2 comparison
In our statistical analysis comparisons, many Middle Eastern studies that used the SF36v2 only report their 0–100 results. Most do not report the scale range (bottom to top). While potentially useful for understanding internal differences between patients, the lack of data on a standardized general population limited what these studies in the Middle East could report. Because the research studies we used for comparison only published 0–100 scores and not norm-based scoring, it was difficult to interpret the significance of differences in scoring.
Palestinian SF36v2 scores were generally lower across all domains (Table 7), except compared with older Italian patients with thalassaemia in a 2008 study in Milan where Palestinian thalassaemia patients scored better for social functioning and role emotional (27). Because the Italian study did not report standard deviations, we do not know whether this difference was statistically significant. Compared with the results of another Italian study in 2008, the only significant difference with our results was for bodily pain, with Palestinian patients having lower scores (28). Compared with the results of a 2021 Italian study, Palestinians in our study had significantly lower scores than Italian patients for lower bodily pain, general health, vitality and social functioning (29). Because these data are not norm-based scores, we do not know if any of the scores are clinically significant.
The 2012 European Evaluation of Patients’ Iron Chelation (EPIC ) trial on the oral chelator deferasirox only reported 0–100 scores for each SF36v2 domain and graphically showed score ranges (15). The study did however report the physical component summary and mental component summary scores (and SDs) which requires norm-based scores for each domain. Both summary scales show that thalassaemia patients in the EPIC study had significantly better overall quality of life than patients in Palestine. It is important to note that EPIC patients were generally from high-income countries or from established clinics with access to adequate resources.
It is theoretically possible to convert published 0–100 data to norm-based scoring. However, because we lacked necessary information such as maximum and minimum scores, any such conversion will not accurately reflect the data set and remains strictly theoretical (see the difference in reported and calculated scores for the mental health component and physical health component in the 2012 EPIC trial (15). This exercise however can give us an “eyeball test” of the clinical effect of thalassaemia on quality of life. We used the SF36v2 recommended norm-based scoring score of < 47 as being below average for the general population and suggesting clinical significance. As shown in the theoretical comparisons in Table 7, Palestinian patients had scores < 47 in all domains, suggesting poor quality of life of clinical significance. The data also show that even in high-income countries where patients have access to more health care resources, the psychosocial health of thalassaemia patients is severely affected (scores < 47 for mental health component, social functioning, role emotional and mental health). With the exception of the 2008 studies in Italy (27,28), thalassaemia also affects the physical heath component score, with lower scores for role physical and general health. A 2021 study in Greece shows the effect of new oral chelators on quality of life with improved mental health scores (29), while the Italian data (29) show improved social functioning scores.
The SF36v2 norm-based scoring scores suggests that, while Palestinian psychosocial scores are low, they are also low for thalassaemia patients in other countries. This suggests that the disease’s pathophysiology and needed clinical interventions create a level of patient concern that affect their health outcomes. On the physical health side, the lower physical functioning and higher bodily pain reported by Palestinian patients are troubling. How a patient reports physical functioning could be associated with access to blood transfusions, because higher haemoglobin levels from transfusion have an immediate response. Everywhere, patients with access to health care resources report greater physical functioning and less bodily pain. The low bodily pain scores suggest that Palestinian patients are under-transfused. Within the context of conflict areas, restricted access to blood transfusion is not surprising and would lead to lower patient quality of life scores in all domains.
Our study has some limitations. We used a convenience sample, time to gather the data was limitedand supporting clinical data were lacking. Several barriers were noted such as difficulty for participants to reach clinic sites, and lack of funding. There are also challenges associated with gathering paediatric patient data because most quality of life assessments are conducted through a parental caregiver proxy. The study instruments used are not specific to thalassaemia which is a potential limitation as particular nuances of the disease could be missed.
Conclusion
Our study shows that research can be conducted in areas of conflict with active collaboration between research groups. The results could help health care professionals plan ways to support access to care for people with chronic illness and hence improve quality of life.
Our data suggest that the low quality of life scores could be associated with patients’ haemoglobin levels which are below the international recommendations (4). The low Palestinian quality of life physical measures are most likely related to low transfusion levels at clinic visits.
Systematic research to collect quality of life data as well as clinical data, including patient outcomes, haemoglobin and iron levels, and complications from treatment, is suggested to strengthen our findings. Research on the geographical distribution of health care under conflict conditions would be enlightening (31,32).
While the patient physical functioning score and physical component summary score were better in 2015 than 2009, the sores in other domains were worse. This may reflect a decline in the Palestine–Israel situation in 2015 and increased conflict, leaving thalassaemia patients having to cope with their disease and limited access to health care under occupation.
References
- Vichinsky EP, MacKlin EA, Waye JS, Lorey F, Olivieri NF. Changes in the epidemiology of thalassemia in North America: a new minority disease. Pediatrics. 2005;116(6):e818–25. https://doi.org/10.1542/peds.2005-0843
- Lal A, Wong T, Keel S, Pagano M, Chung J, Kamdar A, et al. The transfusion management of beta thalassemia in the United States. Transfusion. 2021;61(10):3027–39. https://doi.org/10.1111/trf.16640
- Adam S. Quality of life outcomes in thalassaemia patients in Saudi Arabia: a cross-sectional study. East Mediterr Health J. 2019;25(12):887–96. https://doi.org/10.26719/2019.25.12.887
- Aldwaik R, Abu Mohor T, Idyabi I, Warasna S, Abdeen S, Karmi B, et al. Health status of patients with β-thalassemia in the West Bank: a retrospective-cohort study. Front.Med. 2021:2591. https://doi.org/10.3389/fmed.2021.788758
- Khodashenas M, Mardi, P, Taherzadeh-Ghahfarokhi N, Tavakoli-Far, B., Jamee, M,, Ghodrati N. Quality of life and related paraclinical factors in Iranian patients with transfusion-dependent thalassemia. J Environ Public Health. 2021:2849163. https://doi.org/10.1155/2021/2849163
- Health and well-being profile of the Eastern Mediterranean Region. An overview of the health situation in the Region and its countries in 2019. Cairo: World Health Organization, Regional Office for the Eastern Mediterranean; 2020.
- Sirdah M, Tarazi I, El Jeadi H, Al Haddad R. Normal blood cells reference intervals of healthy adult at the Gaza Strip-Palestine. J Clin Lab Anal. 2008;22(5):353–61. https://doi.org/10.1002/jcla.20265
- Sirdah M, Tarazi I, Al Najjar E, Al Haddad R. Evaluation of the diagnostic reliability of different RBC indices and formaulas in the differentiation of the beta-thalessemia minor from iron deficiency in Palestinian population. Int J Lab Hematol. 2008;30(4):324-30. https://doi.org/10.1111/j.1751-553X.2007.00966.x
- Darwish H, El-Khatib F, Ayesh S. Spectrum of beta-globin gene mutations among thalassemia patients in the West Bank region of Palestine. Hemoglobin. 2005;29(2):119-32. https://doi.org/10.1081/HEM-200058581
- Faraon R, Daraghmah M, Samarah F, Srour M. Molecular characterization of β-thalassemia intermedia in the West Bank, Palestine. BMC Hematol. 2019;19(1):1-9. https://doi.org/10.1186/s12878-019-0135-6
- Sobota A, Yamashita R, Xu Y, Trachtenberg F, Kohlbry P, Kleinert D, et al. Quality of life in thalassemia: A comparison of SF-36 results from the thalassemia longitudinal cohort to reported literature and the US norms. Am J Hematol. 2011;86(1):92. https://doi.org/10.1002/ajh.21896
- Kohlbry P, al-Karmi B, Yamashita R. The quality of life of thalassemia patients living in Palestine, a pilot study. Paper presented at the Thalassemia International Federation’s, 12th International Conference on Thalassemia and Hemoglobinopathies, 11–14 May 2011, Antalya, Turkey (https://www.pagepressjournals.org/index.php/thal/article/view/thal.2011.s1/15, accessed 15 January 2023).
- Rights AMCFH. Israeli forces kill Palestinian and injure 57 in Gaza. Gaza: Al Mezan Center for Human Rights; 2015 (https://www.mezan.org/en/post/21106/Israeli+Forces+Kill+Palestinian+and+Injure+57+in+Gaza, accessed 11 January 2023).
- Kohlbry PA. Plots and deeds: property and formations of land defense in the West Bank. Baltimore, MD: Johns Hopkins University; 2019.
- Porter J, Bowden DK, Economou M, Troncy J, Ganser A, Habr D, et al. Health-related quality of life, treatment satisfaction, adherence and persistence in β-thalassemia and myelodysplastic syndrome patients with iron overload receiving deferasirox: results from the EPIC clinical trial. Anemia. 2012;2012. https://doi.org/10.1155/2012/297641
- Coons SJ, Alabdulmohsin SA, Draugalis JR, Hays RD. Reliability of an Arabic version of the RAND-36 Health Survey and its equivalence to the US-English version. Med Care. 1998;36(3):428–32. https://doi.org/10.1097/00005650-199803000-00018
- Ewing JE, King MT, Smith NF. Validation of modified forms of the PedsQL generic core scales and cancer module scales for adolescents and young adults (AYA) with cancer or a blood disorder. Qual Life Res. 2009;18(2):231–44. https://doi.org/10.1007/s11136-008-9424-4
- Upton P, Eiser C, Cheung I, Hutchings HA, Jenney M, Maddocks A, et al. Measurement properties of the UK-English version of the Pediatric Quality of Life Inventory™ 4.0 (PedsQL™) generic core scales. Health Qual Life Outcomes. 2005;3(1):1–7. https://doi.org/10.1186/1477-7525-3-22
- Varni J, Seid M, Rode CA. The PedQL measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126–39. https://doi.org/10.1097/00005650-199902000-00003
- Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL™ family impact module: preliminary reliability and validity. Health Qual Life Outcomes. 2004;2(1):55. https://doi.org/10.1186/1477-7525-2-55
- Karmi DB. Thalessemia Patients Friends’ Society, Palestine 2022. Ramallah: Thalessemia Patients Friends’ Society, Palestine; 2022 (https://tpfs.ps/lang/en, accessed 26 December 2022).
- Tarazi I, Al Najjar E, Lulu N, Sirdah M. Obligatory premarital tests for beta-thalassaemia in the Gaza Strip: Evaluation and recommendations. Int J Lab Hematol. 2007;29(2):111–8. https://doi.org/ 10.1111/j.1751-553X.2006.00836.x
- Tuysuz G, Tayfun F. Health-related quality of life and its predictors among transfusion-dependent thalassemia patients. J Pediatr Hematol Oncol. 2017;39(5):332–6. https://doi.org/10.1097/MPH.00000000000000790
- Boonchooduang N, Louthrenoo O, Choeyprasert W, Charoenkwan P. Health-related quality of life in adolescents with thalassemia. Pediatr Hematol Oncol. 2015;32(5):341–8. https://doi.org/10.3109/08880018.2015.1033795
- Caocci G, Efficace F, Ciotti F, Roncarolo MG, Vacca A, Piras E, et al. Health related quality of life in Middle Eastern children with beta-thalassemia. BMC Blood Disord. 2012;12(1):6–12. https://doi.org/10.1186/1471-2326-12-6
- Jastrowski Mano KE, Khan KA, Ladwig RJ, Weisman SJ. The impact of pediatric chronic pain on parents’ health-related quality of life and family functioning: reliability and validity of the PedsQL 4.0 Family Impact Module. J.PediatrPsychol. 2011;36(5):517–27. https://doi.org/10.1093/jpepsy/jsp099
- Messina G, Colombo E, Cassinerio E, Ferri F, Curto R, Altamura C, et al. Pyschosocial aspects of psychiatric disorders in young adult with thalassemia major. IEM. 2008;3:339–43. https://doi.org/10.1007/s11739-008-0166-7
- Scalone L, Mantovani LG, Krol M, Rofail D, Ravera S, Grazia Bisconte M, et al. Costs, quality of life, treatment satisfaction and compliance in patients with β-thalassemia major undergoing iron chelation therapy: the ITHACA study. CurrMedRes Opin. 2008;24(7):1905–17. https://doi.org/ 10.1185/03007990802160834
- Tedone F, Lamendola P, Lopatriello S, Cafiero D, Piovani D, Forni GL. Quality of life and burden of disease in Italian patients with transfusion-dependent beta-thalassemia. J Clin Med. 2021;11(1):15. https://doi.org/10.3390/jcm11010015
- Goulas V, Kouraklis-Symeonidis A, Manousou K, Lazaris V, Pairas G, Katsaouni P, et al. A multicenter cross-sectional study of the quality of life and iron chelation treatment satisfaction of patients with transfusion-dependent β-thalassemia, in routine care settings in Western Greece. Qual Life Res. 2021;30(2):467–77. https://doi.org/10.1007/s11136-020-02634-y
- Dewachi O, Skelton M, Nguyen V-K, Fouad FM, Sitta GA, Maasri Z, et al. Changing therapeutic geographies of the Iraqi and Syrian wars. Lancet. 2014;383(9915):449–57. https://doi.org/10.1016/S0140-6736(13)62299-0
- Kohlbry P. Owning the homeland: property, markets, and land defense in the West Bank. J Palest Stud. 2018;47(4):30–45. https://doi.org/10.1525/jps.2018.47.4.30
Decomposing gender disparity in the risk of noncommunicable diseases in adults, Islamic Republic of Iran
Ebrahim Rahimi,1 Rasool Mohammadi,2 Yaser Mokhayeri3 and Seyed S.H. Nazari4
1Department of Public Health, Mamasani Higher Education Complex for Health, Shiraz University of Medical Sciences, Shiraz, Islamic Republic of Iran. 2Department of Biostatistics and Epidemiology, School of Public Health and Nutrition, Lorestan University of Medical Sciences, Khorramabad, Islamic Republic of Iran. 3Cardiovascular Research Center, Shahid Rahimi Hospital, Lorestan University of Medical Sciences, Khorramabad, Islamic Republic of Iran. 4Prevention of Cardiovascular Disease Research Center, Department of Epidemiology, School of Public Health and Safety, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic of Iran. (Correspondence to Seyed S.H. Nazari:
Abstract
Background: Gender disparity in the risk of noncommunicable diseases and its relationship with other social health determinants is not well researched.
Aims: To assess the factors contributing to gender disparity in overall risk of noncommunicable diseases in the Islamic Republic of Iran.
Methods: This study was a secondary analysis of data on about 11 000 adults aged 15–69 years from the 2011 STEPS survey in the Islamic Republic of Iran. The outcome variable in our analysis was the noncommunicable disease risk factor index. We used an extension of the Blinder-Oaxaca decomposition model to decompose the predicted mean difference in this index. Sampling method and study design were taken into account in the analysis. As well as sex, the predictor variables were: age; household assets index; education; work status; ethnicity; and residence.
Results: The overall mean (standard deviation) noncommunicable diseases risk score was 39.26 (22.4). The risk score for women was significantly higher than that for men (41.75 versus 36.84; P < 0.001). About 35% of gender disparity in risk score was due to the different distribution of the predictor variables (explained component); of these, age contributed the most (23.79%), followed by education (7.82%). The different gender effects of work status and age made the largest contributions to the unexplained component of the disparity, 36.40% and 14.82%, respectively.
Conclusions: Policies to reduce the risk of noncommunicable diseases need to consider gender groups and how gender interacts with social determinants such as work status to make some gender subgroups more vulnerable than others.
Keywords: noncommunicable diseases; risk factors; gender equity; Iran.
Citation: Rahimi E; Mohammadi R; Mokhayeri Y; Nazari SSH. Decomposing gender disparity in the risk of noncommunicable diseases in adults, Islamic Republic of Iran. East Mediterr Health J. 2023;29(6). https://doi.org/10.26719/emhj.23.046
Received: 15/09/21; accepted: 21/11/22
Copyright: © Authors; licensee World Health Organization. EMHJ is an open access journal. All papers published in EMHJ are available under the Creative Commons Attribution Non-Commercial ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Introduction
Noncommunicable diseases (NCDs) are the leading cause of both morbidity and mortality worldwide (1). About 71% of all deaths and 43% of the world’s disease burden are attributed to NCDs; these percentages are higher in low- and middle-income countries, with 79% and 85% being reported, respectively (2). The Islamic Republic of Iran is also facing the challenge of NCDs: 83.5% of all deaths and 78.1% of the burden of diseases were attributed to NCDs in 2019 (1).
The rising prevalence of NCDs is caused by unhealthy lifestyles and behavioural changes, especially in developing countries (3). Determining the prevalence of the risk factors for these diseases, combined with effective policy-making for their management, can slow the rising trend (4). In most interventions aimed at promoting health, NCDs risk factors are considered in isolation. However, some NCDs, mainly cardiovascular disease, cancer, chronic respiratory disease and diabetes, share common risk factors. Therefore prevention and control of any of these diseases should integrated into approaches to prevent NCDs as a group. This integrated approach can maximize collaboration and improve efficiency of the prevention and control measures, which is especially important for efficient use of limited resources (5)
Given that these diseases and their risk factors vary by community and population groups (4), their disease burden differs in these groups. Ignoring cultural and social differences when designing control programmes will likely widen health gaps between the populations (2,6). Even though NCDs have received increased attention (7,8), the fact that the contextual conditions for acquiring such diseases are different for each community has been neglected (4,6).
Some studies have shown that social- and gender-related disparities exist in the risk of NCDs (4,9–12). These gender disparities in NCD risk and their relationship with other social health determinants, such as education, residence, employment and socioeconomic status, have not received explicit attention (13–15).
Given the evidence on the different prevalence of most NCDs and their risk factors in men and women in the Islamic Republic of Iran (16,17), this study aimed to assess the contribution of various factors to the gender disparity. Determining the origins of the disparity will help policy-makers develop more effective intervention programmes aimed at reducing the burden of NCDs (18).
Methods
Study design and sample
This study was a cross-sectional analysis of secondary data from the sixth round of the national STEPS survey in Islamic Republic of Iran in 2011. The nationwide survey (primary study) was a cross-sectional, population-based study based on the World Health Organization’s (WHO) STEPwise approach to surveillance of NCD risk factors (19). It included about 12 000 Iranian people aged 6–70 years selected through multistage cluster random sampling with inclusion probability proportional to the population size. In our study we included about 11 000 adults aged 15–69 years. To compensate for over-sampling in some provinces and to increase the accuracy of the estimations, the sampling method and study design were taken into account in the analysis. For this purpose, the data were weighted based on the population.
Outcome variable
The outcome variable was the NCD risk factor index as a proxy for NCD risk score. The risk factors considered for estimating risk score were: insufficient physical activity; low fruit and vegetable consumption; cigarette smoking; overweight and obesity; and high blood pressure. We included these core risk factors because they have the greatest impact on NCD burden, modification is possible through effective intervention and their information can be obtained using questionnaire and simple physical measurements (20). All risk factors were expressed as binary variables according to standard recommendations (21–24). Sufficient physical activity was considered a combination of moderate and vigorous intensity physical activity at least 600 metabolic equivalent of task (MET) minutes per week according to WHO recommendations (21). The general recommendation for daily intake of fruits and vegetables is five or more servings, and this was the cut-off used in our analysis (22). Current and daily use of any type of cigarette (factory-made cigarettes, hand-rolled cigarettes or cigars) was considered current cigarette smoking. Based on body mass index (BMI), participants with a BMI ≥ 25 kg/m2 were classified as overweight and obesity (23). High blood pressure was defined as a systolic blood pressure > 140 mmHg, a diastolic blood pressure > 90 mm Hg, or current use of antihypertensive medication (24).
To account for the different contribution of each risk factor to the burden of NCDs, scoring for each risk factor was weighted proportionate to its contribution (25). That means the presence of each risk factor was scored relative to its contribution to the burden of NCDs, and absence of that the risk factor resulted in a contribution of 0 to the final index. The total risk score for each respondent was calculated from the sum of his/her weighted scores for all risk factors. These scores were then converted into a 0–100 scale.
Predictor variables
In addition to sex (male/female), the potential predictor variables examined were: age (in years); household assets index (see below); highest educational level (no education, < high-school diploma, high-school diploma, higher education (at least some college)); work status (employed, unemployed, non-labour force according to the Bureau of Labour Statistics (26)); ethnicity (Persian/non-Persian) and residence (rural/urban). To determine the household assets index, factors related to the infrastructure and facilities of the home were measured, including access to a dedicated bathroom, kitchen, refrigerator, vacuum cleaner, washing machine and computer. Using polychoric principal component analysis and applying the correlation matrix, the variables with the greatest impact on the total variance were identified. Finally, the first principal component (new variable) that accounted for > 65% of the combined variance was considered the household assets index.
Statistical analysis
Stata, version 14 was used for all analyses. We used descriptive statistics, including central tendency and dispersion indices for quantitative variables and frequencies and percentages for qualitative variables. To decompose the gender disparity in the overall risk of NCDs, an updated package of the Blinder-Oaxaca decomposition model was used (27,28). The mean predicted gender difference in the NCD risk factor index from the model was considered an estimate of the related gender disparity. The model, which is based on a counterfactual regression approach, considers the frequency distribution and differential effect of contributing factors that vary across gender groups, i.e. it decomposes the gender disparity in the overall risk of NCDs into smaller components. Thus, the method explains how much of the disparity in mean predicted outcome (NCD risk factor index) is due to differences in the levels of the related factors in males and females (explained components) and how much is due to gender discrimination, but may also be due the differential effect (the magnitude of regression coefficients) of the factors and other unknown associated factors (unexplained components). In fact, the existence of inequality despite identical individual characteristics can be rooted in unknown factors that affect the outcomes (27,29).
Assuming that the NCD risk score (Y) is explained by n predictors (x1, ... .xn), the gender mean difference in predicted Y(D) is as follows:
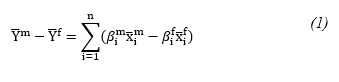
Where x ̅i is the mean value of each predictor variable and β is the predicted regression coefficient.
If the male coefficients are used as the reference, then decomposition is formulated from the viewpoint of females. In other words, the counterfactual equation (βim xi^f) can be obtained by replacing the female coefficients with those of the male equation. By adding and subtracting the counterfactual equation in the right hand side of equation (1), the gender disparity in the mean predicted Y(D) can be decomposed into explained (E) and unexplained (U) components. It is straightforward to show that:
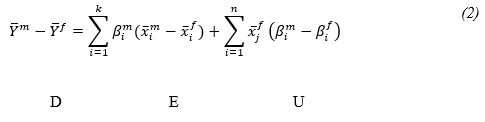
Ethical approval
Approval for the study was obtained from the ethics committee of the Shahid Beheshti University of Medical Sciences. Tehran (IR.SUMS.RETECH.REC.1399.765).
Results
Baseline characteristics
The baseline characteristics of all study participants is given in Table 1. Men comprised about 51.86% of the participants, and most (68.53%) were urban residents. The mean age (standard deviation (SD)) of the participants was 35.89 (SD 14.37) years. In terms of education, 16.71% were not educated, while 46.11% had graduated from high school or had a higher education. Less than half the participants (42.88%) of were part of the labour force, while. 28.45% of men and 86.68% of women were non-labour force. Almost half of the participants (47.94%) were of Persian ethnicity. Significant differences were found between males and females for: age (P= 0.001), household assets index (P= 0.001), educational attainment (P= 0.001), work status (P < 0.001) and type of work (P = 0.001). The mean NCD risk factor index was significantly higher in women (41.75; SD 24.31) than men (36.84; SD 18.78) (P < 0.001).
Low consumption of fruits and vegetables (73.58%) was the leading risk factor, followed by obesity and overweight (49.23%), insufficient physical activity (38.68%), high blood pressure (22.05%) and cigarette smoking (11.53%). Significant gender differences were found in cigarette smoking (men 21.76%; women 1.01%), insufficient physical activity (men 26.73%; women 50.99% and obesity and overweight (men 43.05%; women 55.59%); all P = 0.001.
Decomposition results
The decomposition of the gender disparity in predicted mean NCD risk is shown in Table 2. The disparity was in favour of men (P < 0.001). Only about 35% of the gender disparity was due to the different distribution of the associated factors (explained component). Age contributed the most (23.79%) to the explained component, followed by educational attainment (7.82%). About two thirds of the disparity was attributed to the different effect of the variables (unexplained component), of which about 20.96% was due to unknown variables that were not included in the model. The different effects of work status and age made the largest contribution to the unexplained component, accounting for 36.40 and 14.82%, respectively.
Discussion
We aimed to assess the contribution of related factors to gender disparity in the overall risk of acquiring NCDs in adults living in the Islamic Republic of Iran using data from a nationwide survey. Our findings suggest that men and women have different levels of exposure to NCD risk factors. Moreover, the overall NCD risk for women was higher than for males. Decomposition of the risk showed that work status, educational level and age substantially contributed to this gender difference.
Consistent with other study (12,17,30,31), our study highlights the higher risk of NCDs in women. Evidence from a new WHO report shows that the different gender roles and cultural norms have a major effect on the NCD risks of men and women. These factors tend to change with age and according to social and cultural influences. Accordingly, this leads to different behaviours which affect exposure to NCD risk factors and health and help-seeking behaviour (32).
Despite this higher risk, the focus on women’s health is often limited to reproductive ages and NCDs are considered primarily men’s diseases (12,33). This gender bias could lead to inadequate screening of NCD risk factors in women, making it difficult to control these diseases in women (12). While women tend to live longer than men, older women experience poorer health and more and NCD multimorbidity than older men, with higher associated cost (12,33). This highlights a challenge in health care provision for older women in the Islamic Republic of Iran.
Our findings suggest that the mean age difference between males and females contributed the most to the disparity in NCD risk factor index. In addition, our study indicates that a significant part of the observed disparity was attributed to the different gender effects of age. That means the women experienced greater NCD risk with increasing age than men. This finding is consistent with other studies suggesting multimorbidity is more prevalent in elderly women than in elderly men (16,17,34,35). Of course greater longevity is associated with increased morbidity and women tend to live longer than men. At the same time, elderly women often encounter multiple challenges including financial insecurity, illiteracy, discrimination, domestic violence, physical dependency and a greater possibility of living alone. As well as these difficulties, their health needs and specific requirements are often neglected (33). Therefore, more attention should be given to elderly women in this regard.
Work status also contributed to the observed disparity in our study. This may be due to different jobs and working conditions between men and women in the labour market, which in turn has resulted in different prevalence rates and patterns of health outcomes in men and women (15). According to the Framingham Study, the combination of occupation and family responsibility roles exerted considerable pressure on women. Interpersonal relationships, coping styles and occupations of some working women, along with family responsibilities, have been reported to influence the development of some NCDs in women, such as coronary heart disease (36). This finding is plausible given that the activities generally expected of women in Iranian society involve less physical activity than those associated with men, which is in line with other studies in the Islamic Republic of Iran (29,37). In a gender-biased society, women often have less access to education and social and health services, and fewer employment opportunities than men. Moreover, in Iranian society, education and work settings are also highly gendered which could cause gender inequalities in health. In spite of the substantial increase in women’s enrolment in higher education in the Islamic Republic of Iran, significant development in the women’s labour force has not taken place and they continue to face discrimination in the job opportunities (38).
Furthermore, different gender effects of educational status also contributed to the disparity. Thus, equalizing the educational levels of men and women could potentially reduce the total difference. This finding, together with female educational levels below those of men, suggests that the overall risk of NCDs for women tends to be lower in more highly educated women. This corresponds with study in the Republic of Korea that showed a higher burden of NCDs in less educated groups, especially in women (39); however, it was not the case in an earlier study among Asians (40).
Data for our study were taken from the sixth round of the national STEPS survey in Islamic Republic of Iran, a population-based survey based on the STEPwise approach proposed by WHO (20) and adapted to local conditions. Given the study design, our findings could be generalized to the entire population. However, despite adopting a suitable approach in measuring and explaining gender disparity in NCD risk factors and taking into account the roles of the related social determinants of health, our study had some limitations. First, we used data from a national survey and could not therefore ascertain the reliability of the data. Furthermore, as with other cross-sectional studies, causal relationships between the variables cannot be determined. Therefore, our findings should be used with caution if considering policy-making and intervention design. Future research should focus on determining whether or not the identified correlations are causal in nature, thereby providing the knowledge required for effective control and prevention of NCD risks, especially in women.
Conclusions
Our findings highlight the importance of gender-based population level interventions aimed at preventing NCDs. NCD risk-reduction policies need to consider not only gender groups but also how gender interacts with social determinants to make some gender subgroups more vulnerable than others. Elderly women should be a focus of interventions aimed at reducing gender disparity in the risk of common NCDs. Targeted education about healthy lifestyles in these women and improving their knowledge of NCD risk factors can reduce the risk of these diseases. Women’s occupation is also a fundamental factor in NCD risk. Therefore a women’s information registration system and the use of appropriate key indicators to periodically monitor and evaluate their health is recommended. To this end, further cooperation between government agencies, research institutes and academia related to women’s health issues seems necessary.
Acknowledgements: The authors thank the centre for NCD control of the Iranian Ministry of Health and Medical Education for providing the data for this study.
Funding: None.
Competing interests: None declared.
References
- Azadnajafabad S, Mohammadi E, Aminorroaya A, Fattahi N, Rezaei S, Haghshenas R, et al. Non-communicable diseases’ risk factors in Iran; a review of the present status and action plans. J Diabetes Metab Disord. 2021:1–9. https://doi.org/10.1007/s40200-020-00709-8
- Noncommunicable diseases. Key facts [Internet]. Geneva: World Health Organization (https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases, accessed March 2022).
- Di Cesare M, Khang YH, Asaria P, Blakely T, Cowan MJ, Farzadfar F, et al. Inequalities in non-communicable diseases and effective responses. Lancet. 2013;381(9866):585–97. https://doi.org/10.1016/S0140-6736(12)61851-0
- Allen L, Williams J, Townsend N, Mikkelsen B, Roberts N, Foster C, et al. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: a systematic review. Lancet Glob Health. 2017;5(3):e277–e89. https://doi.org/10.1016/S2214-109X(17)30058-X
- Habib SH, Saha S. Burden of non-communicable disease: global overview. Diabetes Metab Syndr. 2010;4(1):41–7. https://doi.org/10.1016/j.dsx.2008.04.005
- Mathers C, Vos T, Stevenson C. The burden of disease and injury in Australia. Canberra: Australian Institute of Health and Welfare; 1999.
- Daar AS, Singer PA, Persad DL, Pramming SK, Matthews DR, Beaglehole R, et al. Grand challenges in chronic non-communicable diseases. Nature. 2007;450(7169):494–6. https://doi.org/10.1038/450494a
- Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, et al. Priority actions for the non-communicable disease crisis. Lancet. 2011;377(9775):1438–47. https://doi.org/10.1016/S0140-6736(11)60393-0
- Crimmins EM, Kim JK, Sole-Auro A. Gender differences in health: results from SHARE, ELSA and HRS. Eur J Public Health. 2011;21(1):81–91. https://doi.org/10.1093/eurpub/ckq022
- Dash SR, Hoare E, Varsamis P, Jennings GL, Kingwell BA. Sex-specific lifestyle and biomedical risk factors for chronic disease among early-middle, middle and older aged Australian adults. Int J Environ Res Public Health. 2019;16(2):224. https://doi.org/10.3390/ijerph16020224
- Adhikari K, Gupta N, Koshy AK. Gender differences on risk factors of noncommunicable diseases: a community based cross-sectional study in central Nepal. J Nepal Health Res Counc. 2014;12(27):89–93.
- Christiani Y, Byles JE, Tavener M, Dugdale PJ. Gender inequalities in noncommunicable disease risk factors among Indonesian urban populations. Asia Pac J Public Health. 2016;28(2):134–45. https://doi.org/10.1177/1010539515626265
- Eikemo TA, Gkiouleka A, Rapp C, Utvei SS, Huijts T, Stathopoulou T. Non-communicable diseases in Greece: inequality, gender and migration. Eur J Public Health. 2018;28(Suppl 5):38–47. https://doi.org/10.1093/eurpub/cky219
- McNamara CL, Toch-Marquardt M, Albani V, Eikemo TA, Bambra C. The contribution of employment and working conditions to occupational inequalities in non-communicable diseases in Europe. Eur J Public Health. 2021;31(1):181–5. https://doi.org/10.1093/eurpub/ckaa175
- Niedhammer I, Chastang JF, David S, Kelleher C. The contribution of occupational factors to social inequalities in health: findings from the national French SUMER survey. Soc Sci Med. 2008;67(11):1870–81. https://doi.org/10.1016/j.socscimed.2008.09.007
- Khorrami Z, Rezapour M, Etemad K, Yarahmadi S, Khodakarim S, Mahdavi Hezaveh A, et al. The patterns of non-communicable disease multimorbidity in Iran: a multilevel analysis. Sci Rep. 2020;10(1):3034. https://doi.org/10.1038/s41598-020-59668-y
- Alimohammadian M, Majidi A, Yaseri M, Ahmadi B, Islami F, Derakhshan M, et al. Multimorbidity as an important issue among women: results of a gender difference investigation in a large population-based cross-sectional study in West Asia. 2017;7(5):e013548. http://dx.doi.org/10.1136/bmjopen-2016-013548
- Handbook on health inequality monitoring with a special focus on low- and middle-income countries. Geneva: World Health Organization; 2013 (https://apps.who.int/iris/handle/10665/85345, accessed 8 December 2022).
- Noncommunicable disease surveillance, monitoring and reporting. STEPwise approach to NCD risk factor surveillance (STEPS). Geneva: World Health Organization (https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps, accessed 8 December 2022).
- WHO STEPS surveillance manual. The WHO STEPwise approach to noncommunicable disease risk factor surveillance. Geneva: World Health Organization; 2005 (https://apps.who.int/iris/handle/10665/43376, accessed 8 December 2022).
- Noncommunicable diseases and their risk factors: global physical activity questionnaire (GPAQ): analysis guide. Geneva: World Health Organization; 2021 (https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/physical-activity-surveillance, accessed 8 December 2022).
- Boehm JK, Soo J, Zevon ES, Chen Y, Kim ES, Kubzansky LD. Longitudinal associations between psychological well-being and the consumption of fruits and vegetables. Health Psychol. 2018;37(10):959–67. https://doi.org/10.1037/hea0000643
- Defining adult overweight and obesity [Internet]. Atlanta, GA: Centers for Disease Control and Prevention; 2022 (https://www.cdc.gov/obesity/adult/defining.html, accessed May 2021).
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–52. https://doi.org/10.1161/01.HYP.0000107251.49515.c2
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380(9859):2224–60. https://doi.org/10.1016/S0140-6736(12)61766-8
- Bregger JE, Haugen SE. BLS introduces new range of alternative unemployment measures. Monthly Lab Rev. 1995;118:19.
- Jann B. The Blinder-Oaxaca decomposition for linear regression models. Stata J. 2008;8(4):453–79. https://doi.org/10.1177/1536867X0800800401
- Rahimi E, Hashemi Nazari SS. A detailed explanation and graphical representation of the Blinder-Oaxaca decomposition method with its application in health inequalities. Emerg Themes Epidemiol. 2021;18(12):1–15. https://doi.org/10.1186/s12982-021-00100-9
- Rahimi, E, Hashemi-Nazari SS, Etemad K, Soori H. Decomposing gender disparity in total physical activity among Iranian adults. Epidemiol Health. 2017;39:e2017044. https://doi.org/10.4178/epih.e2017044
- Khuwaja AK, Kadir MM. Gender differences and clustering pattern of behavioural risk factors for chronic non-communicable diseases: community-based study from a developing country. Chronic Illn. 2010;6(3):163–70. https://doi.org/10.1177/1742395309352255
- Ahmadi A, Shirani M, Khaledifar A, Hashemzadeh M, Solati K, Kheiri S, et al. Non-communicable diseases in the southwest of Iran: profile and baseline data from the Shahrekord PERSIAN Cohort Study. BMC Public Health. 2021;21(1):2275. https://doi.org/10.1186/s12889-021-12326-y
- New data from WHO/Europe shows links between gender and noncommunicable diseases [Internet]. Copenhagen: World Health Organization, Regional Office for Europe; 2020 (https://www.who.int/europe/news/item/09-12-2020-new-data-from-who-europe-shows-links-between-gender-and-noncommunicable-diseases, accessed May 2022).
- George MS, Gaitonde R, Davey R, Sukumaran V, Mohanty I, Upton P, et al. Social networks and their impact on access to health care: insights from older widows living alone in Kottayam, South India. Ageing Soc. 2021:1–23. https://doi.org/10.1017/S0144686X21001100
- Kim KI, Lee JH, Kim CH. Impaired health-related quality of life in elderly women is associated with multimorbidity: results from the Korean National Health and Nutrition Examination Survey. Gend Med. 2012;9(5):309–18. https://doi.org/10.1016/j.genm.2012.08.001
- Alharbi BA, Masud N, Alajlan FA, Alkhanein NI, Alzahrani FT, Almajed ZM, et al. Association of elderly age and chronic illnesses: role of gender as a risk factor. J Family Med Prim Care. 2020;9(3):1684–90. https://doi.org/10.4103/jfmpc.jfmpc_1060_19
- Ainy E, Azizi FJ. Women, occupation and cardiovascular risk factors: findings from the Tehran Lipid and Glucose Study. Public Health. 2007;121(12):950–3. https://doi.org/10.1016/j.puhe.2006.12.016
- Ramazani Y, Karbasian N, Mobasheri MJ. A Survey on the state of physical activity among middle-aged women in health center in Zarin Shahr City in Iran, fall 2016. J Shahrekord Univ Med Sci. 2018;20(1):15–21 [In Farsi].
- Beyraghi N, Soklaridis S. Toward an understanding of the gender gap in Iran: Why health leaders should care and what they can do to close the gender gap? Iran J Psychiatry Behav Sci. 2019;13(1). https://doi.org/10.5812/ijpbs.64643
- Kim GR, Nam CM. Temporal trends in educational inequalities in non-communicable diseases in Korea, 2007–2015. PLoS One. 2017;12(12): e0190143. https://doi.org/10.1371/journal.pone.0190143
- Ahmed SM, Hadi A, Razzaque A, Ashraf A, Juvekar S, Ng N, et al. Clustering of chronic non-communicable disease risk factors among selected Asian populations: levels and determinants. Glob Health Action. 2009;2(1):1986. https://doi.org/10.3402/gha.v2i0.1986


