Research article
H. Alkhalidi 1 and H. Kfoury 1
حالة من خلل التوافق في جينين ترميميَّيْن hMSH2 وhMSH6 في سرطان المستقيم والقولون لدى المرضى السعوديين: تحليل مناعي نسيجي كيميائي
هشام الخالدي، هالة كفوري
الخلاصـة: تهدف هذه الدراسة إلى التعرف على حالة واسمَيْن رئيسيَّيْن من واسمات عدم الاستقرار في السَّوَاتل المجهرية (وهما جينان ترميميَّان hMSH2 وhMSH6) لدى مرضى سرطان المستقيم والقولون في مستشفى الملك خالد الجامعي، في الرياض، في المملكة العربية السعودية، في الفترة بين 2007 و2009. وأجرى الباحثان تحليلاً مناعياً نسيجياً كيميائياً لمدى عدم الاستقرار في السَّوَاتل المجهرية باستخدام أضداد للجينين hMSH2 وhMSH6. وذلك بتحليل 32 كتلة من مرضى تتراوح أعمارهم بين 16 و83 عاماً (وسطياً 56 عاماً)، أخذت 14 كتلة منها (%43.8) من عمليات استئصال، وأخذت 18 كتلة منها (%56.2) من خزعات. ووجد الباحثون مكوِّناً من الأورام الغدية في أربع كُتَل (%12.5). وقد أبدى كلُّ من سرطانة القولون والأورام الغدية والنسج السليمة تفاعلاً نووياً قوياً للجينَيْن hMSH2 وhMSH6 في %96.9 من الحالات. وبلغ معدل فقدان التعبير %3.1. وكان معدل الطفرة في المجموعة المدروسة الصغيرة منخفضاً، وتَوَافَقَ مع المعدل الوارد في البحوث المنشورة في بلدان صناعية. وتمس الحاجة لمزيد من الدراسات لتأكيد استخدام هذين الواسِمَيْن في تشخيص سرطان المستقيم والقولون.
ABSTRACT This study aimed to identify the status of 2 major microsatellite instability markers (repair genes hMSH2 and hMSH6) in colorectal cancer cases operated at King Khalid University Hospital, Riyadh, Saudi Arabia between 2007 and 2009. Immunohistochemical study of microsatellite instability was done with antibodies to hMSH2 and hMSH6. A total of 32 blocks were analysed from patients aged 16–83 years (median 56 years); 14 blocks (43.8%) were from resections and 18 (56.2%) were from biopsies. An adenomatous component was present in 4 (12.5%) blocks. The colonic carcinoma, the adenomas and the normal tissue showed strong nuclear reactivity to hMSH2 and hMSH6 in 96.9% of the cases. The rate of loss of expression was 3.1%. The rate of mutation in our sampled population was low and matched the rate reported in the literature from industrialized countries. Further studies are needed to confirm the use of these markers in the diagnosis of colorectal cancer.
Statut des gènes d'appariement et de réparation hMSH2 et hMSH6 chez des patients saoudiens atteints d'un cancer colorectal : analyse immunohistochimique
RÉSUMÉ La présente étude visait à identifier le statut de deux marqueurs principaux de l'instabilité des microsatellites (gènes de réparation hMSH2 et hMSH6) dans des cas de cancers colorectaux opérés à l'hôpital universitaire King Khalid de Riyad (Arabie saoudite) entre 2007 et 2009. L'étude immunohistochimique de l'instabilité des microsatellites a été conduite avec des anticorps des gènes hMSH2 et hMSH6. Au total, 32 blocs ont été analysés chez des patients âgés de 16 à 83 ans (âge médian 56 ans) ; 14 blocs (43,8 %) provenaient de résections et 18 (56,2 %) de biopsies. Une composante adénomateuse était présente dans 4 blocs (12,5 %). Le carcinome du colon, les adénomes et les tissus normaux présentaient une forte réactivité nucléaire aux gènes hMSH2 et hMSH6 dans 96,9 % des cas. Le taux de perte d'expression était de 3,1 %. Le taux de mutation dans la population de notre échantillon était faible et correspondait au taux connu dans la littérature des pays industrialisés. Des études supplémentaires sont nécessaires pour confirmer l'utilisation de ces marqueurs dans le diagnostic du cancer colorectal.
1Department of Pathology, College of Medicine, King Saud University, Riyadh, Saudi Arabia (Correspondence to H. Al-Khalidi:
Received: 15/09/11; accepted: 07/12/11
EMHJ, 2012, 18(11):1114-1117
Introduction
Colorectal cancer, the third most common cancer in the world [1], is rare before the age of 40 years and shows a significant age-specific increase in incidence beyond 50 years [2]. Genetics may play a role in up to 30% of cases [3]. Most hereditary colorectal cancers are attributable to 2 recognized syndromes: familial adenomatous polyposis and hereditary non-polyposis colorectal cancer syndrome (HNPCC). HNPCC accounts for the bulk of familial colorectal cancers [4,5] and is known to arise due to mutations in DNA mismatch repair genes [6].
Mismatch repair proteins correct the insertion and deletion mutations that occur when DNA is copied before cell division. Inactivation of one of the mismatch repair genes (hMLH1, hMSH2, hPMS1, hPMS2, GTBP/hMSH6) is responsible for the microsatellite instability or replication error seen in more than 90% of HNPCC cases and 15% of sporadic colorectal cancers [7]. The Bethesda guidelines recommend screening for microsatellite instability or mismatch repair protein immunohistochemistry in patients aged less than 50 years who are genetically predisposed to colorectal cancer [8]. If mismatch repair defects are found, these patients can then be appropriately counselled and further tested for specific gene mutations [6].
In Saudi Arabia, 2 studies found varying percentages from 7% to 37% of patients with colorectal cancer were younger than 50 years of age [9,10] and showed a more aggressive course in comparison with data from an industrialized country population (New Zealand) [11]. Furthermore, another 2 studies from Saudi Arabia explored microsatellite instability using immunohistochemical stains for hMSH2 and hMLH1 and showed a high prevalence of mismatch gene expression loss among Saudis in comparison with that in more developed countries [12,13]. As there may be a hidden familial risk for colorectal cancer, there is a case to be made for a mass screening programme, preferably for individuals aged less than 50 years old. This study was therefore conducted to identify the status of 2 major microsatellite instability markers (repair genes hMSH2 and hMSH6) in colorectal cancer cases operated at our institution over a 3-year period.
Methods
Tissue blocks of 32 different patients with colorectal cancer operated at the King Khalid University Hospital, King Saud University, Riyadh, Saudi Arabia between 2007 and 2009 were retrieved and analysed. Patients’ demographic characteristics including age and sex, the site/s and the type of colorectal cancer were recorded.
Immunohistochemical study of the microsatellite instability was done with antibodies to hMSH2 and hMSH6. Immunohistochemical staining was performed using 4-mm sections of formalin-fixed, paraffin-embedded tissue, which were mounted on capillary gap microscope slides (DAKO ChemMate, A/S BioTek Solutions) and dried at room temperature overnight followed by 1–2 h at 60 °C. The tissue sections were deparaffinized and rehydrated. Antigen retrieval was achieved by microwave treatment in 1 mM EDTA, pH 9.0, at 900 W for 8 minutes followed by 15 min at 350 W. The slides were then allowed to cool for at least 20 min in the EDTA solution. Primary antibodies were mouse monoclonal IgG antibodies to hMSH2 (clone 25D12, dilution 1:50, Novocastra/Leica) and hMSH6 (clone 44, dilution 1:10, ABCAM).
Staining was performed in an automated immunostainer (Bondmax, Leica/ Novocastra), according to the manufacturer’s instructions. DAKO ChemMate kit peroxidase/3, 30-diaminobenzidine was used for hMSH2 and DAKO Envision TM/HRP rabbit/mouse for hMSH6, with rabbit anti-mouse IgG, dilution 1:400, as a link to amplify between the primary antibody and the Envision step. Diaminobenzidine was used as a chromogen. The sections were counterstained with haematoxylin, dehydrated in ascending concentrations of alcohol to xylene and mounted. The Bond polymer refine detection kit was used (catalogue no. DS9800).
Loss of expression of the respective mismatch repair genes protein was defined as absence of nuclear staining in the tumour cells, and normal nuclear staining in lymphocytes and normal epithelial or stromal cells was required serving as internal control. The expression was classified by 2 pathologists as present, absent or non-evaluable without grading of the staining intensity.
Results
The median age of the patients was 56 years (range 16–83 years); 20 (62.5%) were males and 12 (37.5%) were females (male to female ratio 1.67:1). Of the 32 blocks 14 (43.8%) were from resection specimens and 18 (56.2%) were from tissue biopsies. The site of samples was 13 (40.6%) from the rectum, 10 (32.3%) from the sigmoid, 2 (6.3%) from the rectosigmoid, 2 (6.3%) from the hepatic flexure, 2 (6.3%) from the caecum, 1 (3.1%) from the ascending colon, 1 (3.1%) from the transverse colon and 1 (3.1%) from the descending colon. Normal colonic tissue was present in all the blocks except in 2 cases. An adenomatous component was present in 4 (12.5%) blocks (Table 1). The colonic carcinoma, the adenomas, and the normal tissue showed strong nuclear reactivity to hMSH2 and hMSH6 in all the cases, except in 1 case where expression was lost in the carcinoma but was present in the normal adjacent tissue (Figure 1). The rate of loss was 3.1% (1/32).
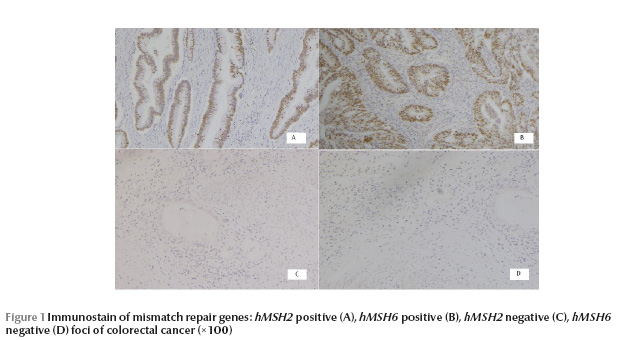
Discussion
In our study, 31 out of 32 of the cases were normal for hMSH2 and hMSH6 and in 1 case expression was lost in the carcinoma and was seen in the normal adjacent tissue. This finding is different from the 2 previous studies on Saudi patients published in 2005 and 2007, which showed an increased rate of mutation in these repair genes [12,13].
Our study showed a low prevalence of mismatch repair genes using the above mentioned 2 markers. There was a strong nuclear reactivity to both hMSH2 and hMSH6 (92.3% to 100%) in all biopsied areas, which is similar to the findings in developed country populations [14,15]. This may be attributed to the fact that since this is a general community hospital, it accepts all types of patients with colorectal carcinoma, thus eliminating selection bias. Furthermore, 80%–90% of mutations in hMSH2 are specific for Lynch syndrome patients; however around 10% of hMSH2 are not, and these are found in families with atypical HPNCC or extracolonic carcinomas [16]. hMLH1 function is context-dependent in low-stress colorectal cancers, and its effect on cell numbers is limited [15]. Most low-level microsatellite instability in colorectal cancers can be explained without requiring an elevated loss of expression during neoplastic development, and hence there is little evidence for a discrete microsatellite instability group of cancers [17]. Nevertheless, our results remain inconclusive because we had only 32 patients. The differences between our findings and previous studies, particularly those conducted among Saudi Arabians, should be further investigated using all 4 markers on a larger sample. Genetic testing on a similar sample is needed.
References
- Howe HL et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among US Hispanic/Latino populations. Cancer, 2006, 107:1711–1742.
- Eddy DM. Screening for colorectal cancer. Annals of Internal Medicine, 1990, 113:373–384.
- Lichtenstein P et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. New England Journal of Medicine, 2000, 343:78–85.
- Vasen HF et al. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Diseases of the Colon and Rectum, 1991, 34:424–425.
- Banerjea A, Clark S, Dorudi S. The changing face of familial colorectal cancer. British Medical Journal, 2005, 330:2–3.
- Aaltonen LA et al. Clues to the pathogenesis of familial colorectal cancer. Science, 1993, 260:812–816.
- Aaltonen LA et al. Replication errors in benign and malignant tumors form hereditary nonpolyposis colorectal cancer patients. Cancer Research, 1994, 54:1645–1648.
- Umar A et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. Journal of the National Cancer Institute, 2004, 96:261–268.
- Aljebreen A. Clinico-pathological patterns of colorectal cancer in Saudi Arabia: Younger with an advanced stage presentation. Saudi Journal of Gastroenterology, 2007, 13:84–87.
- Mansoor I, Zahrani IH, Abdul Aziz S. Colorectal cancers in Saudi Arabia. Saudi Medical Journal, 2002, 23:322–327.
- Isbister WH. Colorectal cancer below age 40 in the Kingdom of Saudi Arabia. Australian and New Zealand Journal of Surgery, 1992, 62:468–472.
- Bavi PP et al. Colorectal carcinomas from Middle East. Molecular and tissue microarray analysis of genomic instability pathways. Saudi Medical Journal, 2008, 29:75–80.
- Al-Kuraya KS et al. Colorectal carcinoma from Saudi Arabia. Analysis of MLH-1, MSH-2 and p53 genes by immunohistochemistry and tissue microarray analysis. Saudi Medical Journal, 2006, 27:323–328.
- Goel A et al. Low frequency of Lynch syndrome among young patients with non-familial colorectal cancer. Clinical Gastroenterology and Hepatology, 2010, 8:966–971.
- Jackson T et al. MLH1 function is context dependent in colorectal cancers. Journal of Clinical Pathology, 2011, 64:141–145.
- Jacob S, Praz F. DNA mismatch repair defects: role in colorectal carcinogenesis. Biochimie, 2002, 84:27–47.
- Graham T et al. Most low-level microsatellite instability in colorectal cancers can be explained without an elevated slippage rate. Journal of Pathology, 2008, 215:204–210.


