Research article
D.I. Abd-Alaleem,1 K.I. Attiaa,1 A.A. Khalefa 1 and R.A. Ahmad2
مستويات الأديبونكتين في مصل السيدات اللاتي يعانين من مقدِّمات الارتعاج
داليا إبراهيم عبد العليم، كاميليا إبراهيم عطية، عبير البيومي عطية خليفة، رضا عبد العزيز أحمد مصطفى
الخلاصـة: يتمتع الأديبونكتين بمفعولٍ مُحَسِّسٍ للأنسولين، ومفعولٍ مضاد للالتهاب، ومفعولٍ مضاد للتعصُّد. إلا أن التقارير بشأن دور الأديبونكتين في مقدِّمات الارتعاج [التشنُّج النفاسي] متضاربة. وقد قام الباحثون في مصر من خلال هذه الدراسة بتقصِّي الترابُط بين مستويات الأديبونكتين في المصل وبين حدوث مقدمات الارتعاج، وكذلك بين مستويات الأديبونكتين وبين بعض الـمُتَثَابتات السريرية والهرمونية. وتم تقسيم عينة تضم ستين سيدة في الأُثلوث الثالث من الحمل إلى ثلاث مجموعات متساوية: حمل طبيعي، مقدمات ارتعاج طفيفة، مقدمات ارتعاج وخيمة. وتبين أن مستويات أديبونكتين المصل لدى السيدات اللاتي يعانين من مقدمات الارتعاج تفوق بشكل يُعتدُّ به إحصائياً مستوياتها في حالات الحمل الطبيعي، وتتضح هذه الزيادة بشكل أكبر في حالات مقدِّمات الارتعاج الوخيمة. ولوحظ ترابط سلبي يُعتدُّ به إحصائياً بين مستويات الأديبونكتين وبين ضغط الدم الشرياني في كل المجموعات. إلا أنه لم يوجد أيُّ نوع من أنواع الترابط بين الأديبونكتين في المصل وبين البيلة البروتينية أو مستويات هرمونَيْ الإيستراديول والبروجستيرون. وهذه النتائج تدعم النظرية القائلة بأن الأديبونكتين قد يكون جزءاً من آلية ارتجاعية تُحَسِّن الحساسية للأنسولين والصحة القلبية الوعائية في المريضات اللاتي يعانين من مقدمات الارتعاج.
ABSTRACT: Adiponectin has profound insulin-sensitizing, anti-inflammatory and anti-atherogenic effects. However, reports of the role of adiponectin in pre-eclampsia are conflicting. This study in Egypt investigated the association between serum adiponectin levels and pre-eclampsia and between adiponectin levels and some clinical and hormonal parameters. A sample of 60 pregnant women in the third trimester were divided into 3 equal groups: normal pregnancy, mild pre-eclampsia and severe pre-eclampsia. Serum adiponectin levels in pre-eclamptic women were significantly higher than in normal pregnant women and the increase was more marked in cases of severe pre-eclampsia. There was a significant negative correlation between adiponectin levels and arterial blood pressure in all groups. However, there was no correlation between serum adiponectin and proteinuria or estradiol and progesterone levels. The results support the theory that adiponectin might be part of a feedback mechanism improving insulin sensitivity and cardiovascular health in pre-eclamptic patients.
Taux sériques d’adiponectine chez les femmes souffrant de prééclampsie
RÉSUMÉ: L’adiponectine a de profonds effets insulino-sensibilisants, anti-inflammatoires et antiathérogènes. Toutefois, les résultats des études sur le rôle de l’adiponectine dans la prééclampsie sont discordants. La présente étude en Égypte a recherché l’association entre les taux sériques d’adiponectine et la prééclampsie et entre les taux d’adiponectine et certains paramètres cliniques et hormonaux. Un échantillon de 60 femmes enceintes au troisième trimestre de grossesse a été constitué, lesquelles ont été réparties en trois groupes comptant un nombre égal de participantes : grossesse normale, prééclampsie légère et prééclampsie sévère. Les taux sériques d’adiponectine chez les femmes prééclamptiques étaient nettement supérieurs à ceux des femmes ayant une grossesse normale et l’augmentation était plus marquée dans les cas de prééclampsie sévère. Les taux d’adiponectine étaient fortement et négativement corrélés à la pression artérielle dans tous les groupes. Cependant, aucune corrélation n’a été trouvée entre l’adiponectine sérique et les taux de protéinurie ou d’estradiol et de progestérone. Les résultats appuient la théorie selon laquelle l’adiponectine pourrait jouer un rôle dans le mécanisme de rétroaction qui contribue à l’amélioration de la sensibilité à l’insuline et la santé cardio-vasculaire des patientes prééclamptiques.
EMHJ, 2011, 17(7): 575-581
1Department of Physiology; 2Department of Obstetrics and Gynaecology, Faculty of Medicine, University of Zagazig, Zagazig, Egypt (Correspondence to R.A. Ahmad:
Introduction
Pre-eclampsia, which occurs in about 5% of pregnancies and results in substantial maternal and neonatal morbidity and mortality, is a pregnancy-specific syndrome of reduced organ perfusion secondary to vasospasm and endothelial activation [1,2]. It is characterized by hypertension and proteinuria.
The syndrome is usually associated with maternal obesity and shares common features with obesity, e.g. hypertension, glucose intolerance, insulin resistance [3] and hyperlipidaemia [4]. Several studies have therefore focused on the relation between pre-eclampsia and hormones secreted by adipocytes [5–7]. Adiponectin is an adipocyte-derived plasma protein [8] that is believed to be involved mainly in the regulation of insulin resistance and glucose homeostasis [9]. Experimental and clinical studies have indicated that low plasma levels of adiponectin are associated with obesity-related metabolic and vascular diseases [10–14], both of which are risk factors for pre-eclampsia. Insulin resistance is a hallmark of pregnancy that peaks in the third trimester [15]. Moreover, this insulin resistance is increased in pregnancies complicated with pre-eclampsia [16].
The evidence that adiponectin interacts with many risk factors of pre-eclampsia, e.g. insulin resistance, inflammatory disorders and abnormal vascular reactivity [17–24], suggests that adiponectin may play a role in the syndrome. However, reports about the levels of adiponectin in pre-eclampsia are conflicting. The present study in Egypt was therefore designed to examine the association between serum adiponectin levels and pre-eclampsia and to detect any correlation between serum levels of this hormone and some clinical and hormonal parameters.
Methods
Sample
A sample of 60 pregnant women in the third trimester of pregnancy were recruited from the outpatient clinics and inpatient department of obstetrics and gynaecology at Zagazig University hospitals, Zagazig, Egypt during the period August 2007 to April 2008. Women targeted were: 20–35 years old, single pregnancy, 20 weeks or more gestational age.
Consent was obtained from each patient after full explanation of the objectives and procedures of the study. Women included in this study were divided into the following groups: normal pregnancy (n = 20), mild pre-eclampsia (n = 20) and severe pre-eclampsia (n = 20). The normal pregnancy (n = 20) and mild pre-eclampsia groups had no medical disorders and normal routine laboratory investigations.
Patients were defined according to criteria established by the Royal College of Obstetricians and Gynaecologists (RCOG) [25]. All women in the normal group were healthy, normotensive and had single gestation. Mild pre-eclampsia was systolic blood pressure > 140 mmHg and/or diastolic blood pressure > 90 mmHg on 2 occasions at least 6 hours apart, typically occurring after 20 weeks gestation (no more than 1 week apart) in conjunction with proteinuria of 300 mg/24h urine collection or > 1+ on 2 random sample urine dipsticks at least 6 hours apart (no more than 1 week apart) [25]. Severe pre-eclampsia was persistent systolic blood pressure > 170 mmHg on 2 occasions or diastolic blood pressure > 110 mmHg on 2 occasions, together with significant proteinuria (at least 1 g/L). Clinical features of severe pre-eclampsia (in addition to hypertension and proteinuria) were: symptoms of severe headache, liver tenderness, visual disturbance, platelet count falling to below 100 × 103/µL epigastric pain and/or vomiting, abnormal liver enzymes (aspartamine or alanine aminotransferase rising to above 70 IU/L), signs of clonus, HELLP syndrome [haemolytic anaemia/elevated liver enzymes/low platelet count] and papilloedema.
All the women included in the study were taking a multivitamin supplement with iron, and none was receiving antihypertensive medications or exogenously administered hormones. None of the participants had any history of hypertension or diabetes.
Data collection
All pre-eclamptic pregnant women were hospitalized, while for the normal pregnant group samples were collected during routine antenatal visits. In all groups gestational age was derived from the last menstrual period and was corrected according to transabdominal and/or transvaginal ultrasonography if needed.
In all groups blood pressure was recorded, body weight and height were measured and body mass index (BMI) was calculated by using the formula of kg/m2 [26]. Blood pressure was measured with the patients in a seated position and with the cuff of the sphygmomanometer at the level of the heart.
A urine sample was taken and proteinuria was determined according to the method of Dilena et al. [27]. A venous blood sample was withdrawn at 09.00 hours following overnight fasting and with the woman in the resting position. Samples were taken into standard serum tubes, allowed to clot, then serum was separated by centrifugation and stored at –60 °C until assayed. The following hormonal assays were carried out: serum adiponectin level according to Arita et al. [10]; serum estradiol level according to Tietz [28]; and serum progesterone level according to Tietz [28].
Statistical analysis
Data are presented as mean and standard error of the mean (SE). Statistical significance was determined by 1-way analysis of variance (ANOVA) test. P values less than 0.05 were considered to be statistically significant. The correlations between the parameters were analysed using Spearman rank correlation. SPSS for Windows, version 10.0 was used for statistical analysis
Results
Serum adiponectin
Table 1 shows the serum adiponectin levels in the 3 study groups. In the mild and severe pre-eclamptic women, the mean serum adiponectin levels [18.8 (SE 0.7) µg/mL and 22.2 (SE 0.7) µg/mL respectively] were significantly higher than that of normal pregnant women [8.5 (SE 0.6) µg/mL] (P < 0.001). Moreover, the mean values in cases of severe pre-eclampsia were significantly higher when compared with those of mild pre-eclampsia (P < 0.01).
Table 1 Serum adiponectin levels in the 3 study groups of pregnant women
BMI
Table 2 shows the BMI of the 3 studied groups. The mean values of BMI in the women with normal pregnancy, mild pre-eclampsia and severe pre-eclampsia [32.2 (SE 0.5) kg/m2, 33.7 (SE 0.7) kg/m2 and 33.8 (SE 0.6) kg/m2 respectively] showed no significant difference between the groups (P > 0.05).
Table 2 Body mass index (BMI) in the 3 study groups of pregnant women
Hormone assays
Table 3 shows the serum levels of estradiol and progesterone in the 3 studied groups. There were no significant difference in estradiol levels between the groups; the mean values in normal pregnancy, mild pre-eclampsia and severe pre-eclampsia were 33.0 (SE 1.4) ng/mL, 33.2 (SE 1.4) ng/mL and 33.0 (SE 1.8) ng/mL respectively (P > 0.05). There were also no significant difference in progesterone levels between the groups; mean values were 105.6 (SE 2.8) ng/mL, 105.3 (SE 2.9) ng/mL and 106.9 (SE 2.9) ng/mL respectively in normal pregnancy, mild pre-eclampsia and severe pre-eclampsia (P > 0.05).
Table 3 Serum levels of estradiol and progesterone in the 3 study groups of pregnant women
Table 4 Correlations between body mass index (BMI) and estradiol and progesterone levels with adiponectin levels
Correlations of serum adiponectin levels and blood pressure
Figures 1 shows that there were negative correlations between serum adiponectin levels and systolic and diastolic blood pressure respectively in all the 3 groups: r = –0.96 and –0.92 (P < 0.001) in the normal pregnancy group (Figure 1a); r = –0.94 and –0.84 (P < 0.001) in the mild pre-eclampsia group (Figure 1b); and r = –0.98 and –0.95 (P < 0.001) in the severe pre-eclampsia group (Figure 1c).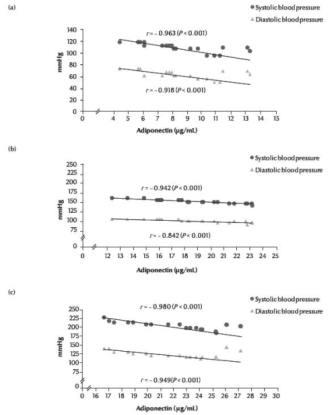
Figure 1 Correlation between serum adiponectin levels and blood pressure in women with (a) normal pregnancy, (b) mild pre-eclampsia, (c) severe pre-eclampsia
Discussion
Our results revealed that serum adiponectin levels were significantly higher in the women with eclampsia in comparison with normal pregnant women who had similar gestational age and BMI. The difference was more marked in cases of severe pre-eclampsia than of mild pre-eclampsia. However, there were no significant differences between the groups in either serum estradiol or progesterone levels.
Our results are supported by those of Davis et al. who reported a significant increase in adiponectin in pre-eclamptic women without any significant changes in serum levels of estrogen, progesterone or human chorionic gonadotrophin [29]. The results are also in line with the findings of D’Anna et al. who reported increases in the levels of adiponectin during the third trimester in pre-eclamptic patients [30].
Several mechanisms to explain the elevation of adiponectin levels in pre-eclampsia have been proposed. It may be secondary to exaggerated non-specific adipocyte lipolysis or a physiological response to enhance fat utilization and attenuated endothelial damage [31]. Other factors that may cause the elevation of plasma adiponectin concentrations in pre-eclampsia include adiponectin resistance [32], alterations in renal function and ongoing adiponectin synthesis in adipose tissues [35].
Serum adiponectin levels did not correlate with BMI, proteinuria, estradiol or progesterone levels in any group. On the other hand, there was a significant negative correlation between serum levels of adiponectin and both systolic and diastolic blood pressure in the pre-eclamptic group.
Our finding that adiponectin levels did not correlate with maternal BMI is in line with the study of Herse et al. [33]. This can be explained by the evidence that BMI in pregnancy does not accurately reflect fat stores because maternal weight is influenced by the weight of the fetus, placenta and amnions and by plasma volume [34]. However, Hendeler et al. concluded that women with severe pre-eclampsia and BMI > 25 kg/m2 have decreased serum adiponectin levels, while normal weight women with pre-eclampsia have increased adiponectin levels [35]. They suggested that the increased adiponectin in normal weight pre-eclamptic women may represent the normal physiological feedback response, but that this mechanism might not function properly in overweight and obese pre-eclamptic woman because of increased adiponectin levels and insulin resistance, suggesting that decreased adiponectin levels may play a role in the pathophysiology of pre-eclampsia.
It is reported that the second half of pregnancy is a state of physiological insulin resistance, which is characterized by hyperinsulinaemia, glucose intolerance and lipid abnormalities [36–41]. This state of insulin resistance is exacerbated in pre-eclampsia, together with enhanced systemic inflammatory reactivity and endothelial dysfunction. At the same time, the circulating levels of both adiponectin and leptin are elevated in pre-eclampsia with the enhancement of insulin sensitivity [15,36–40].
When the pathological characteristics of pre-eclampsia and the changes in adiponectin and leptin levels are considered together, it is clear that the elevation of serum adiponectin and leptin is paradoxical and may play an important role in the pathophysiology of pre-eclampsia. This concept was supported by Fasshauer et al., who reported increased serum levels of adiponectin in pre-eclamptic patients which were positively associated with markers of insulin sensitivity in those patients. They concluded that adiponectin might be part of a physiological feedback mechanism improving insulin sensitivity and cardiovascular health in pre-eclamptic patients [42].
In the present study serum adiponectin levels showed a significant negative correlation with both systolic and diastolic blood pressure in all groups. This finding is in line with that of Li et al. who reported a negative correlation between adiponectin and blood pressure in normotensive populations [43]. Moreover, Ouchi et al. concluded that this effect may be mediated via inflammatory pathway or lipid metabolism [44]. Adiponectin has been shown to have pro-angiogenic, anti-atherogenic and anti-inflammatory functions in the endothelium [45]. Moreover, hypoadiponectinaemia is associated with impaired endothelium-dependent vasodilatation and reduced blood flow [23], and is an independent risk factor for hypertension [44]. These data suggest that adiponectin might maintain endothelial function and its deficiency might lead to endothelial dysfunction/hypertension. Thus, the elevation of circulating adiponectin might be a physiological response to the endothelial dysfunction caused by angiogenic factors derived from the placenta in pre-eclamptic women [46,47]. Taken together, these findings indicate that adiponectin has a role, either direct or indirect in the regulation and integrity of the vascular system.
On the other hand, our results contradict those of other investigators who reported decreased adiponectin levels in pre-eclamptic women in comparison with normotensive pregnant women; they also suggested that this decrease may contribute to the pathophysiology of pre-eclampsia [48–51]. Ouyang et al. found reduced adiponectin levels in pre-eclamptic women, especially in severe cases [52]. This contradictory may relate to differences in body fat, insulin sensitivity or haemoconcentration in the study populations.
In conclusion, adiponectin was markedly increased in case of pre-eclampsia, especially in severe cases, and there was a negative correlation between adiponectin levels and blood pressure, but no correlation between adiponectin levels and BMI, proteinuria, estradiol or progesterone levels. From a preventive and therapeutic perspective, understanding the mechanisms involved in the regulation of adiponectin levels in pre-eclampsia may allow the design of a new class of agents to combat many risk factors in this disease such as hypertension and endothelial dysfunction.
References
- Hypertensive disorders in pregnancy. In: Cunningham FG et al., eds Williams obstetrics, 22nd ed. New York, McGraw-Hill, 2005:761–808.
- Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet, 2001, 357:53–56.
- Fuh MM et al. Resistance to insulin-mediated glucose uptake and hyperinsulinemia in women who had preeclampsia during pregnancy. American Journal of Hypertension, 1995, 8:768–771.
- Hubel CA et al. Fasting serum triglycerides, free fatty acids, and malondialdehyde are increased in preeclampsia, are positively correlated, and decrease within 48 hours post partum. American Journal of Obstetrics and Gynecology, 1996, 174:975–982.
- Laivuori H et al. Evidence of high circulating testosterone in women with prior preeclampsia. Journal of Clinical Endocrinology and Metabolism, 1998, 83:344–347.
- Mise H et al. Augmented placental production of leptin in preeclampsia: possible involvement of placental hypoxia. Journal of Clinical Endocrinology and Metabolism, 1998, 83:3225–3229.
- Williams MA et al. Pre-eclampsia disrupts the normal relationship between serum leptin concentrations and adiposity in pregnant women. Paediatric and Perinatal Epidemiology, 1999, 13:190–204.
- Matsuda M et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. Journal of Biological Chemistry, 2002, 277:37487–37491.
- Mazaki-Tovi S, Kanety H, Sivan E. Adiponectin and human pregnancy. Current Diabetes Reports, 2005, 5:278–281.
- Arita Y et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and Biophysical Research Communications, 1999, 257:79–83.
- Hotta K et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arteriosclerosis, Thrombosis, and Vascular Biology, 2000, 20:1595–1599.
- Yamauchi T et al. Inhibition of RXR and PPARgamma ameliorates diet-induced obesity and type 2 diabetes. Journal of Clinical Investigation, 2001, 108:1001–1013.
- Ouchi N et al. Obesity, adiponectin and vascular inflammatory disease. Current Opinion in Lipidology, 2003, 14:561–566.
- Trujillo ME, Scherer PE. Adiponectin—journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. Journal of Internal Medicine, 2005, 257:167–175.
- Solomon CG, Seely EW. Brief review: hypertension in pregnancy: a manifestation of the insulin resistance syndrome? Hypertension, 2001, 37:232–239.
- Sattar N et al. Leptin levels in pregnancy: marker for fat accumulation and mobilization? Acta Obstetricia et Gynecologica Scandinavica, 1998, 77:278–283.
- Arita Y et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation, 2002, 105:2893–2898.
- Matsuda M et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. Journal of Biological Chemistry, 2002, 277:37487–37491.
- Kobayashi H et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circulation Research, 2004, 94:e27–e31.
- Engeli S et al. Association between adiponectin and mediators of inflammation in obese women. Diabetes, 2003, 52:942–947.
- Ouchi N et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension, 2003, 42:231–234.
- Shand BI et al. Plasma adiponectin in overweight, nondiabetic individuals with or without insulin resistance. Diabetes, Obesity and Metabolism, 2003, 5:349–353.
- Tan KCB et al. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. Journal of Clinical Endocrinology and Metabolism, 2004, 89:765–769.
- Fernández-Real JM et al. Adiponectin is associated with vascular function independent of insulin sensitivity. Diabetes Care, 2004, 27:739–745.
- Habli M, Sibai BM. Hypertensive disorders of pregnancy. In: Gibbs RS, Karlan BY, eds. Danforth’s obstetrics and gynecology, 10th ed. Philadelphia, Lippincott Williams & Wilkins, 2008:258–275.
- McCarthy JF, Misra DN, Roberts JM. Maternal plasma leptin is increased in preeclampsia and positively correlates with fetal cord concentration. American Journal of Obstetrics and Gynecology, 1999, 180:731–736.
- Dilena BA, Penberthy LA, Fraser CG. Six methods for determining urinary protein compared. Clinical Chemistry, 1983, 29:553–557.
- Tietz NW, ed. Clinical guide to laboratory tests, 3rd ed. Philadelphia, WB Saunders, 1995:509–512.
- Davis GK et al. Predicting transformation from gestational hypertension to preeclampsia in clinical practice: a possible role for 24 hour ambulatory blood pressure monitoring. Hypertension in Pregnancy, 2007, 26:77–87.
- D’Anna R et al. Adiponectin and insulin resistance in early- and late-onset pre-eclampsia. British Journal of Obstetrics and Gynaecology, 2006, 113:1264–1269.
- Ramsay JE et al. Paradoxical elevation in adiponectin concentrations in women with preeclampsia. Hypertension, 2003, 42:891–894.
- Kajantie E et al. Adiponectin concentrations in maternal serum: elevated in preeclampsia but unrelated to insulin sensitivity. Journal of the Society for Gynecologic Investigation, 2005, 12:433–439.
- Herse F et al. Circulating and uteroplacental adipocytokine concentrations in preeclampsia. Reproductive Sciences, 2009, 16:584–590.
- Ozkan S et al. Serum leptin levels in hypertensive disorder of pregnancy. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 2005, 120:158–163.
- Hendler I et al. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. American Journal of Obstetrics and Gynecology, 2005, 193:979–983.
- Caminos JE et al. Expression and regulation of adiponectin and receptor in human and rat placenta. Journal of Clinical Endocrinology and Metabolism, 2005, 90:4276–4286.
- Wolf M et al. First trimester insulin resistance and subsequent pre-eclampsia: a prospective study. Journal of Clinical Endocrinology and Metabolism, 2002, 87:1563–1568.
- Sattar N et al. Classic and novel risk factor parameters in women with a history of pre-eclampsia. Hypertension, 2003, 42:39–42.
- Mazaki-Tovi S, Kanety H, Sivan E. Adiponectin and human pregnancy. Current Diabetes Reports, 2005, 5:278–281.
- Sagawa N et al. Role of leptin in pregnancy. Placenta, 2002, 23:S80.
- Ouchi N et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation, 2000, 102:1296–1301.
- Fasshauer M et al. Circulating high molecular weight adiponectin is upregulated in pre-eclampsia and is related to insulin sensitivity and renal function. European Journal of Endocrinology, 2008, 158:197–201.
- Li J et al. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circulation Research, 2005, 97:872–879.
- Ouchi N, Shibata R, Walsh K. Targeting adiponectin for cardioprotection. Expert Opinion on Therapeutic Targets, 2006, 10:573–581.
- Yamauchi T et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. Journal of Biological Chemistry, 2003, 278:2461–2468.
- Davison JM et al. New aspects in the pathophysiology of preeclampsia. Journal of the American Society of Nephrology, 2004, 15:2440–2448.
- Nien JK et al. Adiponectin in severe preeclampsia. Journal of Perinatal Medicine, 2007, 35:503–512.
- Ichida K et al. Plasma adiponectin concentrations and placental adiponectin expression in pre-eclamptic women. Gynecological Endocrinology, 2007, 23:238–243.
- Nakatsukasa H et al. Circulating leptin and angiogenic factors in preeclampsia patients. Endocrine Journal, 2008, 55:565–573.
- Ouyang YQ et al. Plasma sFLt-1 to PIGF ratio is correlated with inflammatory but not with oxidative stress in Chinese preeclamptic women. Archives of Gynecology and Obstetrics, 2008, 280:91–97
- Girouard J et al. Previous hypertensive disease of pregnancy is associated with alterations of markers of insulin resistance. Hypertension, 2007, 49:1056–1062.
- Ouyang Y, Chen H, Chen H. Reduced plasma adiponectin and elevated leptin in pre-eclampsia. International Journal of Gynaecology and Obstetrics, 2007, 98:110–114.


