Research article
A.M. Alkhunaizi,1 S.S.A. Shah,2 U.S. Wesslen,2 Z.A. Al Sadah3 and A. Antony4
الإصابة الحادة في الكلية بعد جراحة القلب في المنطقة الشرقية من المملكة العربية السعودية
أحمد منصور الخنيزي، سيد شاهكار شاه، أولف سفن ويسلن، زينب علوي السادة، أملراج أنتوني
الخلاصـة: تعد الإصابة الحادة في الكلية إحدى المضاعفات الخطيرة لجراحة القلب. وقد أجريت هذه الدراسة لتحديد تواتُر هذه الإصابة وعوامل الاختطار المصاحبة لها بعد إجراء جراحة في القلب، في مركز الظهران الصحي في المنطقة الشرقية من المملكة العربية السعودية. وقد أدرج الباحثون في الدراسة جميع المرضى الذين أجريت لهم جراحة في القلب بين شهرَيْ حزيران/يونيو 2005 وكانون الأول/ديسمبر 2008. وقد استوفى 293 مريضاً منهم معايير الدراسة وأدرجوا في التحليل النهائي لها، وتبين أن 85 مريضاً )29.0%( قد أصيبوا إصابة حادة في الكلية. وعندما أُجري التحليل المتعدِّد ات تبين أن العوامل التي يُعتدُّ إحصائياً بارتباطها بالإصابة الحادة في الكلية هي العمر، والسكّريّ، والمرض الكلوي المزمن السابق للجراحة، ِّريالمتغ مل الحالات، و42.9% في الحالات المحتاجة إلى الدِّيَال. وعلى ُجوالجراحة الطارئة. وكان معدَّل الوفيات المرتبطة بالإصابة الحادة في الكلية 10.5% في م هذا، فإن الإصابة الحادة في الكلية والتالية لجراحة القلب تعتبر مشكلة خطيرة بين المرضى في المنطقة الشرقية من المملكة العربية السعودية. ومن الضروري اتخاذ التدابير للوقاية من هذه المضاعفة.
ABSTRACT: Acute kidney injury is a serious complication after cardiac surgery. This study was conducted to determine the frequency of acute kidney injury and the associated risk factors following cardiac surgery at Dhahran health centre in eastern Saudi Arabia. All patients who underwent cardiac surgery between June 2005 and December 2008 were included. Of 293 patients who fulfilled the criteria and were included in the final analysis, 85 (29.0%) developed acute kidney injury. Using multivariate analysis, the factors significantly associated with acute kidney injury were age, diabetes, preoperative chronic kidney disease and emergent surgery. Mortality associated with acute kidney injury was 10.5% overall and 42.9% when dialysis was required. Acute kidney injury following cardiac surgery is a serious problem among patients in eastern Saudi Arabia. Measures to prevent this complication are essential.
Lésion rénale aiguë après chirurgie cardiaque dans l’est de l’Arabie saoudite
RÉSUMÉ: Une atteinte rénale aiguë représente une complication grave après une chirurgie cardiaque. La présente étude a été conduite afin de déterminer la fréquence des atteintes rénales aiguës et les facteurs de risque associés après une chirurgie cardiaque à l’hôpital de Dhahran dans l’est de l’Arabie saoudite. Tous les patients ayant eu une cardiochirurgie entre juin 2005 et décembre 2008 ont été inclus dans l’étude. Sur 293 patients répondant aux critères et inclus dans l’analyse finale, 85 (29,0 %) souffraient d’une atteinte rénale aiguë. Les facteurs fortement associés à une atteinte rénale aiguë, dégagés aux moyens d’une analyse multivariée, étaient l’âge, un diabète, une maladie rénale chronique préopératoire et une chirurgie d’urgence. Le taux de mortalité associé à une atteinte rénale aiguë était de 10,5 % toutes catégories confondues et de 42,9 % en cas de dialyse. Les atteintes rénales aiguës après une chirurgie cardiaque constituent un problème grave chez les patients dans l’est de l’Arabie saoudite. Des mesures destinées à prévenir cette complication sont essentielles.
EMHJ, 2011, 17(6): 495-500
1Internal Medicine Services Division; 2Surgical Services Division; 3Nursing Services Department; 4Epidemiology Services Unit, Preventive Medicine Services Division, Dhahran Health Centre, Dhahran, Saudi Arabia (Correspondence to A.M. Alkhunaizi:
Introduction
Acute kidney injury (AKI) is a serious complication following cardiac surgery affecting up to 30% of patients [1–3]. It is associated with a high morbidity and mortality, especially when renal replacement therapy is required [4–6]. Some studies have documented that even a small decline in glomerular filtration rate may have a detrimental impact on patients’ outcome [7].
The prevalence and risk factors of AKI among the Saudi population are not known. Most of the earlier studies from developed countries that addressed AKI after cardiac surgery used a rather gross definition of AKI, such as an increase of serum creatinine level ≥ 50% above baseline or the need for renal replacement therapy. In this study in a major hospital in eastern Saudi Arabia we looked at the incidence of AKI following cardiac surgery using the stricter definition of the Acute Kidney Injury Network (AKIN) [8,9] and evaluated some of the risk factors associated with the development of AKI. Risk stratification prior to cardiac surgery should predict the development of AKI with the hope of preventing the condition and/or modifying its course.
Methods
Study design
A case–control study was carried out on 325 patients who underwent open-heart surgery at Dhahran health centre in eastern Saudi Arabia between June 2005 and December 2008. The data were collected using the anaesthesia services division’s database and the computerized patient database at Dhahran health centre. The database was created to record information about all patients who underwent open-heart surgery, including: type of surgery, number of grafts used, cardiopulmonary bypass time and aortic crossclamp time. The great majority of patients studied were Saudi citizens drawn from different tribes in Saudi Arabia. Exclusion criteria were: death within 24 hours postoperatively, incomplete patient data, preexisting renal failure requiring dialysis, a baseline serum creatinine ≥ 4 mg/dL or reoperation: 32 patients did not meet the criteria and were excluded from the analysis, leaving 293 patients who were included in the final analysis.
Definitions
The primary outcome was the development of AKI during the first postoperative week. All patients had their serum creatinine level measured preoperatively. Acute kidney injury was defined as an increase in serum creatinine of ≥ 0.3 mg/dL above baseline that persisted for more than 48 hours. Serum creatinine was checked on admission to the surgical intensive care unit and repeated at least every 24 hours. Serum creatinine was measured by enzymatic assay (Viros 5.1/FS, Ortho-Clinical Diagnostics). Chronic kidney disease was defined as a chronic elevation of serum creatinine ≥ 1.5 mg/dL preoperatively. Left ventricular dysfunction (LVD) was defined as an ejection fraction of ≤ 40%, assessed preoperatively by echocardiography, radiocontrast ventriculography or radio nucleotide ventriculography. Chronic obstructive pulmonary disease (COPD) was defined as functional disability and/or hospitalization, chronic bronchodilator therapy or nicotine smoking of ≥ 20 packs-years. Patients were considered to have diabetes mellitus if they were using insulin and/or oral hypoglycaemic agents at the time of surgery. Operative mortality was defined as death during the index hospitalization.
The following variables were studied: age, sex, presence of diabetes mellitus, presence of chronic kidney disease, presence of underlying LVD, presence of COPD, type of cardiac surgery [coronary artery bypass graft (CABG), valve surgery, combined CABG and valve procedures and other procedures such as ventricular aneurysm repair, pericardiectomy, etc.] and whether the surgery was performed electively or as an emergent procedure. Surgery was performed either off-pump or on cardiopulmonary bypass according to our institutional standards.
Statistical analysis
The association between baseline and intraoperative variables, and the development of AKI were assessed by logistic regression. Variables that were significantly associated with the development of AKI were also included in a multivariate logistic model. Means were expressed as ± 1 standard deviation (SD). Odds ratios (OR) were expressed together with 95% confidence interval (CI) and associated P-values. A P-value of < 0.05 was considered significant. The statistical software SPSS, version 15.0, was used for the statistical analyses.
Results
Background characteristics of the cohort
The great majority of the 293 patients were Saudi citizens (269, 91.8%) with a mean age of 59 (SD 15) years, range 14–80 years. There were 224 (76.5%) male patients [mean age 58.4 (SD 12.0) years, range 14–80 years] and 69 females (23.5%) [mean age 63.0 (SD 9.8) years, range 43–81 years] (Table 1). The clinical characteristics of the patients showed that 192 (65.5%) had diabetes mellitus and 43 (14.7%) had LVD. A total of 18 patients (6.1%) had underlying chronic kidney disease and 83 (28.3%) had underlying COPD.
Table 1 Baseline and intraoperative variables associated with acute kidney injury after cardiac surgery
The operative characteristics of the sample showed that the great majority (272, 92.8%) were elective cases for heart surgery; only 21 (7.2%) were emergent cases (Table 1). Most (266, 90.8%) had coronary artery bypass grafting and 27 (9.2%) had other surgeries. There were 282 patients (96.2%) who had surgery performed on pump, while 11 (3.8%) had the surgery done off-pump. The mean cardiopulmonary bypass time was 109 (SD 152) min and the mean aortic crossclamp time was 66 (SD 46) min.
Variables associated with acute kidney injury
AKI was diagnosed in 85 patients (29.0%) out of the entire cohort. Among the different variables age, presence of diabetes mellitus, LVD and chronic kidney disease were found to be associated with a high risk of developing AKI (Table 1). In addition, AKI was more common among patients who underwent emergent surgery and or had a prolonged bypass time. The mean age of patients who developed AKI was 63 years compared with 58 years in the rest of the group.
Using multivariate analysis, age (OR = 1.68, 95% CI: 1.30–2.20), presence of diabetes mellitus (OR = 0.47, 95% CI: 0.24–0.91), chronic kidney disease (OR = 0.15, 95% CI: 0.05–0.50) and emergent surgery (OR = 0.21, 95% CI: 0.07–0.61) were independently associated with the development of AKI (Table 2). There was a linear association between age and risk of developing AKI (Figure 1). Seven patients (2.4%) required renal replacement therapy, either as haemodialysis or continuous renal replacement therapy.
Table 2 Multivariate analysis of risk factors associated with the development of acute kidney injury following cardiac surgery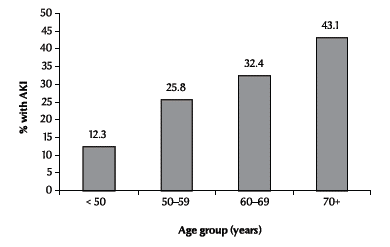
Figure 1 Acute kidney injury (AKI) after surgery by patient’s age
A total of 9 patients died, corresponding to an overall mortality rate of 3.1%. Among patients who developed AKI, mortality was 9/85 (10.5%). Mortality among patients who had renal replacement therapy was even higher at 3/7 (42.9%).
Discussion
Dhahran health centre is one of the main hospitals in Saudi Arabia’s Eastern province, providing primary and/or tertiary care to around 400 000 individuals. The cardiac surgery programme, established in 2005, is a new addition to the services of the health centre. This study was performed to evaluate the development of AKI after cardiac surgery, to identify the risk factors that lead to AKI among our patient population and to compare this with international standards. Although the best measure of renal function is glomerular filtration rate, this is not practical to perform in the context of AKI. There are currently no sensitive and specific markers to detect renal injury in clinical practice, and serum creatinine remains the most used indicator of renal injury.
The reported incidence of AKI after cardiac surgery varies in the literature, with a range of 1%–30%, depending on the definition of AKI. Chertow et al. analysed data from 42 773 patients who underwent cardiac surgery and found an overall incidence of AKI requiring dialysis of 1.1%. The incidence of AKI requiring dialysis in patients who had valvular and combined CABG/valvular surgery was higher at 1.7% and 3.3% respectively [6]. Most of the earlier studies that addressed AKI used a loose definition, such as a rise in serum creatinine of 1.0 mg/dL or greater above baseline, a 50% increase in serum creatinine or the need for renal replacement therapy [1–3,5,6,10–22]. Unlike our study, these studies most likely excluded patients who had a lesser degree of renal dysfunction. There is emerging evidence that a minor increase in serum creatinine is associated with adverse outcomes [12,23–25]. In our study we chose a rather strict criterion for AKI with a cutoff increase in serum creatinine of ≥ 0.3 mg/dL above baseline, in accordance with the recommendations of the AKIN [8,9]. This explains the relatively high incidence of AKI in our population (29.0%) compared with other studies [1–3,5,6,10–22]. There is, however, a genetic variation in the susceptibility of individuals to renal injury [26,27]. Some investigators have found that in patients undergoing cardiac surgery, carriers of the APOE e4 allele had a decreased risk of AKI compared with non-carriers [28,29]. Until now, our knowledge of the genetic polymorphism of AKI is still limited. In the future, a better characterization of genetic predisposition to AKI may enhance risk prediction. Is the Saudi population at increased risk for developing AKI compared with other nations? This is not clear since we do not have data about the genetic makeup of our population, and this is an important area to explore in future studies.
The pathogenesis of renal injury following cardiac surgery is not well understood. It has been divided into preoperative events, intraoperative events and postoperative events; all are related either to impaired renal perfusion or decreased renal reserve [19]. Both haemodynamic and inflammatory factors interact at a cellular level that ultimately lead to the development of acute tubular necrosis, which is manifested as a rise in serum creatinine and decreased urine output. Several risk factors have been identified as being associated with the development of AKI after cardiac surgery, such as female sex, presence of COPD, diabetes mellitus, peripheral vascular disease, renal insufficiency and congestive heart failure, valve surgery, need for emergent surgery, cardiogenic shock requiring intra-aortic balloon, left main coronary artery disease, length of cardiopulmonary bypass, crossclamp time, off-pump versus on-pump surgery, nonpulsatile flow, haemolysis and haemo-dilution [19,30–32]. In our study, several but not all of these factors were found to be associated with the development of AKI. Both population size and patient characteristics may have contributed to the difference between our findings and those reported in earlier studies.
There is a high morbidity and mortality associated with AKI after cardiac surgery. Depending on the definition of AKI and the postoperative period studied (whether time to hospital discharge or 30 days mortality), the mortality rate has been shown to range from 15%–30% [19]. Among our patients who developed AKI, the mortality rate was 10.5%, considerably less than what has been reported in other studies [19]. In addition to the high morbidity and mortality associated with AKI, the cost of treatment of this complication, especially when renal replacement therapy is required, is very high.
Despite all the research in the field, there is currently no effective treatment for AKI, with the exception of supportive measures that include identification of high-risk patients, optimization of renal perfusion and avoidance of nephrotoxins [19]. Pharmacological treatment with atrial natriuretic peptide, fenoldopam, N-acetylcysteine, statins and clonidine have only shown modest results in small trials [19,33]. Therefore it is important to identify and recognize the risk factors that predispose individuals to AKI in order to take the necessary measures to prevent it or modify its course. Several groups have developed clinical scoring systems to help predict the risk for AKI after cardiac surgery [31,32,34]. In our study we identified certain factors that led to the development of AKI: age, diabetes mellitus, chronic kidney disease and emergent surgery. Other factors previously reported to be associated with a higher incidence of AKI, e.g. peripheral vascular disease and COPD, were not found to be significant in our population, possibly due to the small number of patients included in this study.
This study has several limitations, including the single-centre data, the relatively small number of patients and the retrospective observational design. However, the study sheds light on some of the risk factors that lead to AKI among the Saudi population, with the hope that timely recognition of these conditions will lead to better surveillance and monitoring, which may impact positively on the overall outcome of patients undergoing open-heart surgery.
Acknowledgements
The authors acknowledge the use of Saudi Aramco Medical Services Organization (SAMSO) facilities for the research data used in this article. The opinions expressed in this article are those of the authors and not necessarily of SAMSO.
References
- Bellomo R. Defining, quantifying, and classifying acute renal failure. Critical Care Clinics, 2005, 21:223–237.
- Bellomo R, Raman J, Ronco C. Intensive care unit management of the critically ill patient with fluid overload after open heart surgery. Cardiology, 2001, 96:169–176.
- Haase M et al. Cardiopulmonary bypass-associated acute kidney injury: a pigment nephropathy? Contributions to Nephrology, 2007, 156:340–353.
- Alkhunaizi AM, Schrier RW. Management of acute renal failure: new perspectives. American Journal of Kidney Diseases, 1996, 28:315–328.
- Zanardo G et al. Acute renal failure in the patient undergoing cardiac operation. Prevalence, mortality rate, and main risk factors. Journal of Thoracic and Cardiovascular Surgery, 1994, 107:1489–1495.
- Chertow GM et al. Independent association between acute renal failure and mortality following cardiac surgery. American Journal of Medicine, 1998, 104:343–348.
- Lassnigg A et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. Journal of the American Society of Nephrology, 2004, 15:1597–1605.
- Mehta RL et al.; Acute Kidney Injury Network. Report of an initiative to improve outcomes in acute kidney injury. Critical Care (London, England), 2007, 11:R31.
- Molitoris BA et al.; Acute Kidney Injury Network working group. Improving outcomes of acute kidney injury: report of an initiative. Nature Clinical Practice. Nephrology, 2007, 3:439–442.
- Bellomo R et al. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI). International Journal of Artificial Organs, 2008, 31:166–178.
- Dimov V et al. Who is at risk for developing acute renal failure after surgery? IMPACT consults. Proceedings of the 2nd Annual Cleveland Clinic Perioperative Medicine Summit. Cleveland Clinic Journal of Medicine, 2006, 73 (Electronic Suppl. 1):S12–S13.
- Gruberg L et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. Journal of the American College of Cardiology, 2000, 36:1542–1548.
- Hoste EA et al. The epidemiology of cardiac surgery-associated acute kidney injury. International Journal of Artificial Organs, 2008, 31:158–165.
- Kochi AC et al. Preoperative risk factors for the development of acute renal failure in cardiac surgery. Revista Brasileira de Cirurgia Cardiovascular; Orgao Oficial da Sociedade Brasileira de Cirurgia Cardiovascular, 2007, 22:33–40.
- Bellomo R et al.; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care (London, England), 2004, 8:R204–R212.
- Lombardi R, Ferreiro A. Risk factors profile for acute kidney injury after cardiac surgery is different according to the level of baseline renal function. Renal Failure, 2008, 30:155–160.
- Palomba H et al. Acute kidney injury prediction following elective cardiac surgery: AKICS score. Kidney International, 2007, 72:624–631.
- Ronco C, Kellum JA, Bellomo R. Cardiac surgery-associated acute kidney injury. International Journal of Artificial Organs, 2008, 31:156–157.
- Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet, 2005, 365:417–430.
- Schetz M et al. Prevention of cardiac surgery-associated acute kidney injury. International Journal of Artificial Organs, 2008, 31:179–189.
- Tolwani A et al. Treatment of patients with cardiac surgery associated-acute kidney injury. International Journal of Artificial Organs, 2008, 31:190–196.
- Vaschetto R, Groeneveld AB. An update on acute kidney injury after cardiac surgery. Acta Clinica Belgica. Supplementum, 2007, (2):380–384.
- Chertow GM et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. Journal of the American Society of Nephrology, 2005, 16:3365–3370.
- Levy MM et al. Early changes in organ function predict eventual survival in severe sepsis. Critical Care Medicine, 2005, 33:2194–2201.
- Praught ML, Shlipak MG. Are small changes in serum creatinine an important risk factor? Current Opinion in Nephrology and Hypertension, 2005, 14:265–270.
- Lu JC et al. Translational Research Investigating Biomarkers and Endpoints for Acute Kidney Injury (TRIBE-AKI) Consortium. Searching for genes that matter in acute kidney injury: a systematic review. Clinical Journal of the American Society of Nephrology, 2009, 4:1020–1031.
- Haase-Fielitz A et al. Genetic polymorphisms in sepsis- and cardiopulmonary bypass-associated acute kidney injury. Contributions to Nephrology, 2007, 156:75–91.
- Chew ST et al. Preliminary report on the association of apolipoprotein E polymorphisms, with postoperative peak serum creatinine concentrations in cardiac surgical patients. Anesthesiology, 2000, 93:325–331.
- Isbir SC et al. Genetic polymorphisms contribute to acute kidney injury after coronary artery bypass grafting. Heart Surgery Forum, 2007, 10:E439–E444.
- Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Medicine, 2007, 33:409–413.
- Thakar CV et al. A clinical score to predict acute renal failure after cardiac surgery. Journal of the American Society of Nephrology, 2005, 16:162–168.
- Chertow GM et al. Preoperative renal risk stratification. Circulation, 1997, 95:878–884.
- Kandula P. Statins in perioperative prevention of acute kidney injury in patients undergoing cardiac surgery. European Heart Journal, 2009, 30:250.
- Eriksen BO, Hoff KR, Solberg S. Prediction of acute renal failure after cardiac surgery: retrospective cross-validation of a clinical algorithm. Nephrology, Dialysis, Transplantation, 2003, 18:77–81.


