Research article
M.A.A. Wadi 1 and S.S. Al-Sharbatti2
علاقة وزن المولود بتعرُّض الأمهات قسرياً لدخان المدخنين الآخرين
مها وادي، شذى الشربتي
الخلاصـة: أُجريَتْ هذه الدراسة لتقييم علاقة وزن المولود بتعرُّض والدته للتدخين القسري )السلبي(، مع استقصاء بعض المحدِّدات الأخرى لوزن المولود. وقد أُجريَتْ هذه الدراسة الاستعادية في مدينة بغداد بالعراق فيما بين شهري شباط/فبراير وآب/أغسطس 2004 على عينة عشوائية مكوّنة تَّْ مقابلتهنّ في غضون أربع وعشرين ساعة بعد الولادة: مئة وخمسون أماً لم يتعرّضن قسرياً لدخان َممن ثلاثمئة من ربات البيوت غير المدخنات، وت المدخنين في المنزل ومئة وخمسون أمّاً تَعَرَّضْنَ له. وقد كان متوسط وزن المواليد الذين تعرّضت أمّهاتهم للتدخين أقل بدرجة يُعْتَدُّ بها مقارنةً بمواليد .) r = -غير المتعرّضات للتدخين. وقد لوحظت علاقة عكسية يُعْتَدُّ بها بين وزن الوليد وعدد السجائر التي يدخنها أفراد الأسرة )معامل الارتباط 0.27ات الشواهد – أن وزن المولود كان مترابطاً ترابطاً عكسياً بدرجة يُعْتَدُّ ّريات المدروسة على متغّريوأظهر التحليل التَّحَوُّفيّ المتعدد - بعد ضبط جميع المتغبها مع تعرُّض الأمهات للتدخين القسري ولوجود رعاية ملائمة قبل الحمل.
ABSTRACT: This study aimed to assess the relationship between birth weight and maternal exposure to passive smoking during pregnancy, and to investigate some other determinants of birth weight. A retrospective cohort study in Baghdad, Iraq was conducted during February to August 2004 on a random sample of 300 non-smoker housewife mothers, interviewed 24 hours after delivery: 150 were not exposed to passive smoking at home and 150 were exposed. The mean birth weight of exposed newborns was significantly lower than non-exposed newborns. In exposed newborns, a significant inverse relationship was noticed between birth weight and the number of cigarettes smoked by household members (r = –0.27). Multiple regression analysis showed that after controlling for all the variables studied, birth weight had a significant inverse correlation with the maternal exposure to passive smoking and a positive correlation with adequate antenatal care.
Association entre le poids de naissance du nouveau-né et l’exposition au tabagisme passif de la mère à son domicile
RÉSUMÉ: La présente étude a évalué l’association entre le poids de naissance du nouveau-né et l’exposition au tabagisme passif de la mère pendant la grossesse, et a recherché d’autres déterminants du poids de naissance. Une étude de cohorte rétrospective a été conduite à Bagdad, en Iraq, entre février et août 2004 sur un échantillon sélectionné au hasard de 300 mères au foyer non fumeuses, interrogées 24 heures après l’accouchement : 150 mères n’avaient pas été exposées au tabagisme passif à leur domicile, contrairement aux 150 autres femmes. Le poids de naissance moyen des nouveau-nés exposés était significativement inférieur à celui des nouveau-nés non exposés. Chez les nouveau-nés exposés, une relation inverse significative a été observée entre le poids de naissance et le nombre de cigarettes fumées par les membres du ménage (r = –0,27). L’analyse de régression multiple a indiqué qu’après le contrôle de toutes les variables étudiées, le poids de naissance était inversement et fortement corrélé au tabagisme passif, mais positivement et fortement corrélé au recours à des soins prénatals appropriés.
EMHJ, 2011, 17(4): 290-296
1Ministry of Health, Baghdad, Iraq. 2Department of Community Medicine, College of Medicine, Gulf Medical University, Ajman, United Arab Emirates (Correspondence to S.S. Al-Sharbatti:
Introduction
Although tobacco smoke has long been linked to various diseases among smokers, it was not until 1964 that this association was publicly established by the landmark report of the US Surgeon General [1]. Twenty years later, attention turned to the possible effect of tobacco smoke on non-smokers [2]. Passive smoking is involuntary or forced smoking when non-smokers inhale environmental tobacco smoke [3–5].
Maternal exposure to tobacco smoke has been associated with increased levels of nicotine and cotinine in the serum or urine of the mother and the neonate and in the amniotic fluid [6]. These substances constitute a hazard to the fetus as they cross the placental barrier and may act to inhibit fetal growth [6–8]. Maternal passive and light active smoking has been associated with significant reductions in birth weight, crown–heel length, upper- and lower-arm length and head circumference of neonates [9]. The relation between maternal smoking and fetal development is thought to arise from a direct toxic effect of smoke or from an indirect effect mediated by a reduction in maternal weight gain. The relation between birth weight and passive smoking via the father and/or other household members is more difficult to explain; one theory is that smoke inhaled passively has the same effect on the fetus as maternal smoking [10].
No previous studies on this subject have been conducted in Baghdad, Iraq. Hence, the aim of the current study was to assess the relationship between birth weight and home exposure to passive smoking during pregnancy, and to ascertain the influence of selected factors on neonatal birth weight.
Methods
A retrospective cohort study was conducted during February–August 2004.
Sample
The study included a convenience sample of 300 non-smoker housewife mothers who delivered in the labour ward of Al-Yarmok hospital, Baghdad. Only women who were housewives were selected in order to overcome the confounding effect of possible exposure at the work place.
The women were interviewed within 24 hours after delivery. A systematic random sampling method was used to select the women. None of the women invited refused to participate in the study. The study included 2 groups of 150 women: the first group were non-smoker mothers who were not exposed to passive smoking at home during pregnancy; the second group were non-smoker mothers who had a history of smoke exposure.
Mothers’ home exposure to passive smoking was defined as 5 or more cigarettes smoked per day by others in the mother’s presence [11]. Women who were healthy (no history of acute or chronic diseases), had accurate information about their last menstrual period, age and prepregnancy weight, had a singleton pregnancy and gave birth to a full-term baby with no obvious congenital anomalies were included in this study.
Data collection
Direct interview of the participants was done by the research team using a specially designed questionnaire which included the following information: mother’s age and residence, parents’ level of education (primary school or less; intermediate or secondary school; college or higher education), number of household members and number of rooms (used for calculation of crowding index). Parents’ education and crowding index were used as indicators for socioeconomic status. Information about the current pregnancy was collected (gestation; last menstrual period) and expected date of delivery was determined depending on information concerning the last menstrual period. Adequacy of antenatal care was evaluated depending on the number of visits to a primary health care centre (adequate = 5 or more visits during the whole pregnancy [12]). Data about the smoking habit of the child’s father and the smoking habit of all household members in the presence of the pregnant woman were collected.
The birth weight of the baby and the mother’s weight before delivery were measured and weight gain during pregnancy was calculated by subtraction of the prepregnancy weight from the predelivery weight. Mother’s height was also measured and recorded.
The study was approved by the Scientific Council of Community and Family Medicine. The study was discussed with the participants and verbal consent was taken from them before enrolment in the study.
Statistical analysis
The data analysis was carried out using SPSS, version 13. The statistical significance of the difference between the mean and standard deviation (SD) values was assessed using the independent sample t-test, and the significance of the association between the frequencies of other variables was assessed using the chi-squared test. Multiple regression analysis was used to control for interrelations between potential predictors of birth weight.
P < 0.05 was used as the cut-off level for statistical significance.
Results
A total of 300 women were included in this study: 150 women not exposed to passive smoking at home and 150 women exposed to passive smoking. The number of low birth weight babies (< 2500 g) in the exposed and non-exposed groups were 17 (11.3%) and 6 (4.0%) respectively. The odds ratio was 3.07 (95% confidence interval: 1.17–8.01), i.e. exposed women had a 3.07 higher risk of having a low birth weight baby (P < 0.05).
The mean birth weight of the passive smoke-exposed babies was significantly lower [3183 (SD 550) g] than that of the non-exposed babies [3381 (SD 531) g] (t = 3.786, P < 0.001) (Table 1).
Table 1 Distribution of neonatal birth weight by sex and maternal passive smoking exposure
In the exposed neonates, a significant inverse relationship was noticed between birth weight and the number of cigarettes smoked by household members (r = –0.27, P = 0.002) (Figure 1).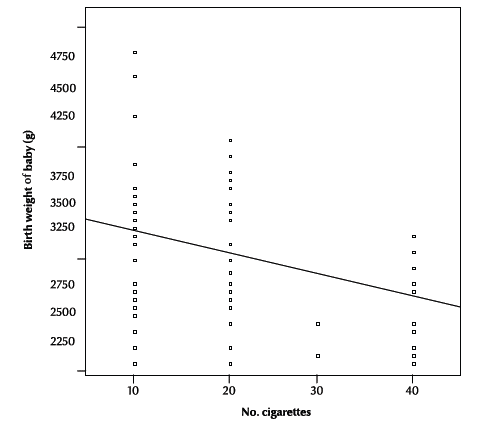
Figure 1 Relationship between birth weight and number of cigarettes smoked by household members (r = –0.27; P = 0.002
The proportion of males and females in the non-exposed babies was 54% and 46% respectively, while for the exposed group it was 35% and 65% respectively (z =3.37, P < 0.05). The mean birth weights were lower in the exposed male and female subgroups compared with their counterparts in the non-exposed groups; the differences were significant for both males and females (Table 1).
Most of the 300 women studied, 86%, resided in urban areas. In these areas the proportion of women exposed to passive smoking was higher than those who were not exposed (52% versus 48%); for women with rural residence the reverse was found (38% versus 62%). No significant association was found between residence and exposure to passive smoking (χ2 = 2.77, P = 0.1). No significant differences were noticed between the mean birth weights of the exposed and non-exposed babies from the rural residence group (Table 2). However, the mean birth weight of the exposed babies in the urban residence group was significantly lower than their counterparts in the non-exposed group.
Table 2 Distribution of neonatal birth weight by residence and maternal passive smoking exposure
Analysis of birth weight according to parents’ education level (Table 3) showed that the mean birth weights were lower in the exposed group for all education levels of mothers and fathers. The differences between the mean birth weights of the exposed and non-exposed groups were significant in neonates whose parents had lower levels of education.
Table 3 Distribution of neonatal birth weight by parents' education level and maternal passive smoking exposure
Concerning history of iron/folic acid supplement intake during pregnancy, the frequency of women who had such a history in the non-exposed and exposed groups were 49.3% and 50.7% respectively (P > 0.05). The mean birth weight values were significantly lower among the smoke exposed versus non-exposed babies, regardless of whether the mothers had or had not received iron/folic acid supplements during their pregnancies (Table 4).
Table 4 Distribution of neonatal birth weight by history of maternal iron/folic acid supplementation and maternal passive smoking exposure
Multiple regression analysis
To control for interrelations between different variables, a multiple regression analysis was done with birth weight as the dependent variable and selected independent variables (Table 5). After controlling for all the variables studied, only passive smoking exposure and utilization of primary health care services (adequate antenatal care) had a statistically significant correlation with birth weight (P < 0.001 and P = 0.03 respectively).
Table 5 Multiple regression model with neonatal birth weight as the dependent variable and selected independent variables
Discussion
The negative effects of passive smoking on the health of the fetus or child continue to receive little attention in the Eastern Mediterranean Region, despite the large volume of research in this area. Passive smoking during pregnancy has been associated with a reduction in head circumference at birth, a higher incidence of sudden infant death syndrome, decreased lung function and increased risk of severe infections, including respiratory syncytial virus bronchiolitis; there is also a relationship between passive smoking and behavioural disorders, including attention deficit/hyperactivity disorder [13].
A significant relationship has been documented between active smoking during pregnancy and fetal growth retardation [14]. Increased interest has also been focused on the effect of passive smoking on birth weight [15]. The present study was conducted to assess the relationship between birth weight and domestic exposure to passive smoking during pregnancy. Selection of the 300 women for the study was based on the history of home exposure to passive smoking during pregnancy.
None of the women in the sample worked outside the home and the estimation of smoke exposure in the current study was done by a questionnaire that involved the number of cigarettes smoked by household members and took into account several potential confounders. Other studies depend on measurement of urine cotinine level to evaluate the risk of exposure to passive smoking [16]. However, a study by the Centers for Disease Control and Prevention found that urine cotinine concentration did not explain more variability in birth weight than did self-reported number of cigarettes smoked per day [17].
Ourt data showed that exposure to passive smoking during pregnancy was associated with a significantly lower mean birth weight in neonates of exposed mothers than those of non-exposed mothers. This observation confirms other reports that passive smoking during pregnancy can adversely affect fetal growth [11,18].
In the present study the group of newborn infants exposed to passive smoking had a mean birth weight 198.4 g lower than non-exposed infants. This is consistent with another study which showed that the birth weight of babies of passive smoking-exposed mothers was 189 g lower than that of babies of the non-exposed mothers [19]. Similarly, a study in Denmark showed that maternal exposure to passive smoking was associated with a reduction in birth weight of 120 g per pack of cigarettes smoked per day by the father. This relationship remained statistically significant after controlling for mother’s age, parity, alcohol and tobacco consumption, illness during pregnancy and sex of the baby [20].
We found that exposure to passive smoking during pregnancy increased the risk of having a low-birth-weight infant (< 2500 g) 3-fold. This finding is consistent with the study in Denmark, in which exposure to passive smoking during pregnancy significantly increased the risk of having a low birth weight infant (relative risk 2.17, 95% CI: 1.05–4.50) [20].
We found a significant inverse relationship between birth weight and number of cigarettes smoked/day by household members. This finding is consistent with that obtained in an intervention controlled study in the Czech Republic, where a strong dose–effect relationship was observed between amount of mothers’ exposure to passive smoking and birth weight [19]. Similarly, the study conducted in Denmark showed a direct relationship between birth weight and average tobacco load when all variables had been controlled in the regression model [20].
The higher frequency exposure to passive smoking in mothers who were urban residents and the significant reduction in the mean birth weight among these mothers noted in our study suggests that the effect of passive smoking on birth weight is more pronounced in urban residents. Consumption of tobacco was less common in rural areas compared with urban areas in a study of smoking and lung cancer in England [10]. Similarly, a prospective cohort study carried out in the United States of America showed that urban populations were more likely to be exposed to passive smoking [21].
The present study showed that the mean birth weight of exposed neonates was lower than that in the non-exposed group, irrespective of their parents’ education levels. The differences between mean values were significant in babies whose parental levels of education were lower. This suggests that the effect of exposure to passive smoking was less pronounced among neonates whose parents’ education levels were higher, a finding which could be attributed to a lower intensity of exposure. In another study done in Poland, the prevalence of smoking was found to be higher among less educated men [22]. Also, a finding of the American Cancer Society study confirmed that passive smoking exposure was greater among households with lower levels of education [23].
In our study, we noted a significantly lower birth weight in the exposed babies, irrespective of maternal intake of iron/folic acid supplements during pregnancy. However, in the unexposed babies, the mean birth weight of those whose mothers took iron/folic acid supplements was slightly higher than that of their counterparts whose mothers did not receive supplements during pregnancy. This is in agreement with the findings of a prospective cohort study in India to evaluate the effect of iron therapy in reducing maternal anaemia and to evaluate the association of maternal haemoglobin and fetal growth. The reasearchers concluded that iron supplementation was an effective mode of treating anaemia among pregnant women, and was inversely associated with fetal growth [24]. Another study in the United Kingdom to evaluate the influence of erythrocyte folate status on birth weight showed that maternal folate intake was a significant predicator of infant birth weight [25].
The multiple regression analysis in this study using birth weight as the dependent variable showed that after the controlling for all variables studied, only passive smoking and antenatal care were associated with birth weight. Passive smoking exposure of mothers during pregnancy was significantly associated with a lower mean birth weight in the exposed group compared with the non-exposed group, and adequate antenatal care for mothers was associated with a significantly higher mean birth weight compared with mothers receiving no antenatal care. Similarly, multiple regression analysis in the Danish study found that paternal smoking remained a significant influence on birth weight after controlling for mother’s age, parity, alcohol and tobacco consumption during pregnancy, illness during pregnancy, social class and sex of the baby [20].
References
- Smoking and health: report of the advisory committee to the Surgeon General of the Public Health Service. Washington DC, US Department of Health Education and Welfare, 1964.
- Adams JD, O’Mara-Adams KJ, Hoffmann D. Toxic and carcinogenic agents in undiluted mainstream smoke and sidestream smoke of different types of cigarettes. Carcinogenesis, 1987, 8:729–731.
- Jamie H. Islamic ruling on smoking. Cairo, World Health Organization, 1996 (http://www.emro.who.int/publications/healthedreligion/Smoking/FT_HamidJamie.htm, accessed 23 January 2011).
- Cook DG et al. Passive exposure to tobacco smoke in children aged 5–7 years: individual, family, and community factors. British Medical Journal, 1994, 308:384–389.
- Guerin MR, Jenkins RA, Tomkins BA. The chemistry of environmental tobacco smoke: composition and measurement. Chelsea, Michigan, Lewis Publishers, 1992.
- Luck W et al. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Developmental Pharmacology and Therapeutics, 1985, 8:384–395.
- Mochizuki M, Maruo T, Masuko K. Mechanism of foetal growth retardation caused by smoking during pregnancy. Acta Physiologica Hungarica, 1985, 65:295–304.
- Mochizuki M et al. Effects of smoking on fetoplacental-maternal system during pregnancy. American Journal of Obstetrics and Gynecology, 1984, 149:413–420.
- Luciano A et al. The influence of maternal passive and light active smoking on intrauterine growth and body composition of the newborn. European Journal of Clinical Nutrition, 1998, 52:760–763.
- Rubin DH et al. Effect of passive smoking on birth weight. Lancet, 1986, 2:415–417.
- Dejmek J et al. The exposure of nonsmoking and smoking mothers to environmental tobacco smoke during different gestational phases and fetal growth. Environmental Health Perspectives, 2002, 110:601–606.
- Gann P, Nghiem L, Warner S. Pregnancy characteristics and outcomes of Cambodian refugees. American Journal of Public Health, 1989, 79:1251–1257.
- Hofhuis W, Merkus PJ, de Jongste JC. Nadelige effecten van passief roken op het (ongeboren) kind [Negative effect of passive smoking on the (unborn) child]. Nederlands Tijdschrift voor Geneeskunde, 2002, 146(8):356–359.
- Matsubara F et al. Maternal active and passive smoking and fetal growth: a prospective study in Nagoya, Japan. Journal of Epidemiology, 2000, 10:335–343.
- Nelson E, Jodscheit K, Guo Y. Maternal passive smoking during pregnancy and fetal developmental toxicity. Part 1: gross morphological effects. Human and Experimental Toxicology, 1999, 18:252–256.
- England LJ et al. Measures of maternal tobacco exposure and infant birth weight at term. American Journal of Epidemiology, 2001, 153:954–960.
- Eskenazi B, Prehn AW, Christianson RE. Passive and active maternal smoking as measured by serum cotinine: the effect on birthweight. American Journal of Public Health, 1995, 85:395–398.
- England LJ et al. Measures of maternal tobacco exposure and infant birth weight at term. American Journal of Epidemiology, 2001, 153:954–960.
- Windham GC et al. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology (Cambridge, Mass.), 2000, 11:427–433.
- Hruba D, Kachlik P. Influence of maternal active and passive smoking during pregnancy on birth weight in newborns. Central European Journal of Public Health, 2000, 8:249–252.
- Perea FP et al. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposure. Neurotoxicology, 2005, 26:573–587.
- Hennekens CH, Buring JE. Epidemiology in medicine, 1st ed. Boston, Massachusetts, Little, Brown, 1987.
- De Ferranti D, Chen J. Curbing the epidemic: governments and the economics of tobacco control, 1st ed. Washington DC, World Bank, 1999.
- Steenland K et al. Environmental tobacco smoke and coronary heart disease in the American Cancer Society CPS-II cohort. Circulation, 1996, 94:622–628.
- Gomber S et al. Impact of daily versus weekly hematinic supplementation on anemia in pregnant women. Indian Pediatrics, 2002, 39(4):339–346


