A. Rahmo1 and M. Hamze 2
التعرف على خصائص المتفطرات السلية بين المرضى السوريين من خلال التفاعل السلسلي للبوليميراز للعناصر المزدوجة المتكررة
منذر حمزة، عبد القادر رحمو، مطانيوس سعادة
الخلاصـة: لم يخضع دور المعالجة المسبقة في ديناميكيات سراية السل لدراسة كافية من قبل. وقد تعرف الباحثون على خصائص مستفردات المتفطرات السلية التي جُمِعَتْ من مرضى سبق أن عولجوا، (وعددهم 88)، ينتمون إلى جميع المناطق في الجمهورية العربية السورية، وذلك من حيث حساسيتها للمضادات الحيوية، ومن حيث أنماطها الجينية، باستخدام طريقة التفاعل السلسلي للبوليميراز للعناصر المزدوجة المتكررة، للتعرف على مدى قرب العناصر المتكررة على الدنا وهذه العناصر هي IS6110 (وهي عنصر جيني متحرك)، وPGRS. وقد أنتجت المستفردات التي يبلغ عددها 88 مستفردةً 59 نمطاً مختلفاً من التفاعلات السلسلية للبوليميراز للعناصر المزدوجة المتكررة. وقد درس الباحثون الروابط المتعلقة بالجنس والعمر والديانة والتحسس والنمط الجيني. وقد أظهرت جميع أرجاء سورية مستويات مرتفعة من التنوع في الأنماط الجينية، مما يشير إلى مستوى منخفض من سراية ذراري المتفطرات السلية لدى المرضى الذين عولجوا سابقاً.
ABSTRACT The role of previous treatment in the dynamics of tuberculosis transmission has not been adequately investigated. Mycobacterium tuberculosis isolates from previously treated patients (n = 88) from all regions of Syrian Arab Republic were characterized in terms of antibiotic sensitivity and genotyping using double-repetitive-element polymerase chain reaction (DRE-PCR) method for the proximity of the repetitive DNA elements IS6110 (a mobile genetic element) and PGRS. The 88 isolates resulted in 59 different DRE-PCR patterns. Correlations related to age, sex, region, sensitivity and genotype were examined. All regions of the country showed high levels of genotype diversity, suggesting a low level of transmission of M. tuberculosis strains in previously treated patients.
Caractérisation de Mycobacterium tuberculosis par PCR d’éléments répétitifs doubles chez des patients syriens
RÉSUMÉ Le rôle des traitements antérieurs dans la dynamique de la transmission de la tuberculose n’a pas été étudié de manière adéquate. Les isolats de Mycobacterium tuberculosis issus de patients précédemment traités (n = 88) provenant de toutes les régions de la République arabe syrienne ont été caractérisés en termes de sensibilité aux antibiotiques et en fonction de leur génotype au moyen de la méthode de PCR d’éléments répétitifs doubles (DRE-PCR) pour la proximité des éléments d’ADN IS6110 répétés (élément génétique mobile) et des séquences répétées PGRS (Polymorphic GC-rich repetitive sequence). Les 88 isolats ont fait apparaître 59 profils DRE-PCR différents. Les corrélations avec l’âge, le sexe, la région, la sensibilité et le génotype ont été étudiées. Une forte diversité des génotypes a été constatée pour toutes les régions du pays, ce qui suggère un faible niveau de transmission des souches de M. tuberculosis chez les patients précédemment traités.
1National Commission for Biotechnology, Damascus, Syrian Arab Republic.
2Faculty of Public Health, Lebanese University, Tripoli, Lebanon (Correspondence to M. Hamze:
Received: 14/01/08; accepted: 29/06/08
EMHJ, 2010, 16(8): 820-830
Introduction
Patients with a previous history of tuberculosis (TB) treatment have been shown worldwide to have the highest risk of harbouring multidrug-resistant (MDR) and even extensively drug-resistant strains of Mycobacterium tuberculosis. There is some evidence that positive selection of strains occurs within patients [1]. However, the role of previous treatment in the dynamics of M. tuberculosis TB transmission and the multitude of factors underlying treatment failure have not been adequately investigated [2].
Molecular studies have been successfully applied in evaluating epidemiological linkages and in the discovery of unexpected ones. These have been essential for distinguishing relapse from reinfection in recurrences of TB [3] and for establishing evidence of laboratory cross-contaminations (false positives) [4] and the association of particular genotypes with hypervirulence and multi-resistance [5].
Different methods have been used for the genotyping of M. tuberculosis TB. These vary in the time required for testing and their resolution, specificity, typeability, reproducibility, robustness, complexity, cost and amenability to databank construction. The double-repetitive-element polymerase chain reaction (DRE-PCR) genotyping method addresses variations in the proximity presence and the interspace distance separating 2 exceptionally important repetitive DNA elements: insertion sequence (IS) 6110 and polymorphic GC rich repeat (PGRS). The method is relatively fast, simple and cost-effective [6]. It suffers, however, from a lack of reproducibility due to the occasional absence of low-intensity bands and from only an average level of resolution [7]. At minimum, the technique serves as a tailored approach for preliminary genotyping and has the potential for efficient implementation in a low-resource country, where TB is generally widespread and endemic.
The presented study in the Syrian Arab Republic was based on isolating various strains of M. tuberculosis TB from previously treated patients from various regions of the country, followed by characterizing their sensitivity to antibiotics and their genotyping through a slightly modified DRE-PCR method. The phenotypic correlation with the genotypes of various strains, based on adjacency of the 2 repetitive elements IS6110 and PGRS, was examined.
Methods
Sample
Following an agreement with the Syrian Ministry of Health, we were able to obtain samples from 88 patients previously treated for pulmonary TB, which included cases of failure after first treatment and of relapse or reinfection. The 88 samples, provided by the Ministry’s central laboratory, were collected between July 2003 and October 2005 from all Syrian provinces (muhafazat). Samples were shipped, accompanied by the related information, to the biomedical laboratory of the National Commission for Biotechnology in the Syrian Arab Republic. The research was approved by the responsible ethical committee at the Ministry of Higher Education.
Specimen preparation and culture
Processing of sputum specimens was based on liquefaction and decontamination by 2% N-acetyl-L-cysteine-NaOH. Bacterial culture was performed on solid Lowenstein–Jensen medium [8].
Susceptibility testing
Antibiotic susceptibility testing was done using the proportion method of the National Committee for Clinical Laboratory Standards [9] on inoculum preparations of freshly grown colonies from Lowenstein–Jensen medium after transfer and resuspension using beads. The patterns of antibiotic sensitivity (S) and resistance (R) are described in the text using the notation RRSR, RRRS etc., with the following sequence: rifampicin (RIF)–isoniazide (INH)–streptomycin (STR)–ethambutol (EMB).
DRE-PCR procedure
For DNA extraction, a loopful of each culture was extracted using the QIAamp DNA blood mini kit (Qiagene). The manufacturer’s instructions were adhered to except for the incubation period, which was extended to 3 hours. The DRE-PCR procedure was followed as reported by Harris [10], a modification of Friedman et al. [11]. The PCR amplification mixture contained 67 mM tris (pH 8.8), 16 mM (NH4)2SO4, 0.01% tween-20 (1 × reaction buffer; Euroclone), 2.5 mM MgCl2 (Euroclone), 200 μM each deoxynucleoside triphosphate (MBI Fermentas), 0.5 μM of each of the 4 primers (MWG Biotech, HPSF grade), and 2.5 U of taq polymerase (Euroclone). The sequence of the primers is described elsewhere [10,11]. Amplification was done according to Harris [10], using the Mastercycler thermal cycler (Eppendorf). The amplification products were analysed using parametric analysis of gene set enrichment (PAGE), stained with ethidium bromide. The modifications introduced were: the use of a DNA extraction kit, the inclusion of ammonium sulfate in the PCR reaction mixture and the use of PAGE for band pattern analysis.
Statistical analysis
SPSS, version 12.0 was used. A confidence interval of 95% (2-tailed) was applied. Cross-tables that did not fulfil Cochrane rules were tested for significance using the Fisher exact test (2-sided). Unpaired Student t-test was used for continuous variables. Correlations were assessed using spearman and Kendall coefficients (rs, τ).
Results
The mean and standard deviation (SD) age of the 88 TB patients was 36.5 (SD 2.7) years, median 34.5, range 17–69 years. There were 63 males, mean age 37.6 (SD 3.2) years (median 35, range 17–69 years) and 25 females, mean age 33.9 (SD 5.0) years (median 35, range 17–62 years) (Table 1). Most isolates belonged to patients in the age range 20–39 years. All patients had been previously treated for M. tuberculosis TB.
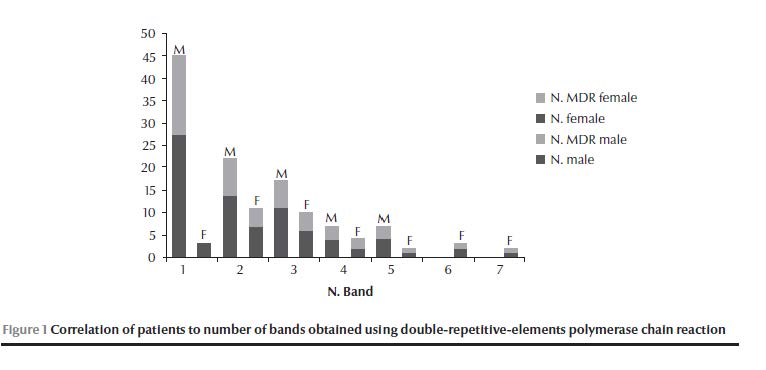
Of the 88 isolates collected, 85 (96.6%) were resistant; 18 (20.5%) were single-resistant, 12 (13.6%) were poly-resistant and 55 (62.5%) were MDR strains. The resistance pattern was: RIF 69.4%, INH 77.6%, STR 82.4% and EMB 61.2%. The most common resistance pattern was RRRR (resistance sequence RIF–INH–STR–EMB) in 45.9% of resistant isolates (39/85). The most common drug resistance was to STR and so the most common single-resistance pattern was SSRS in 16.5% (14/85) of resistant isolates (Table 2). The 88 isolates resulted in 59 different DRE-PCR patterns, while 6 isolates produced no detectable genotypes. The Hunter–Gaston discrimination index [12] was calculated as D = 0.98. The number of different bands was 24, ranging from 1 to 7 for each genotype (Table 3). (Tabel 3 continued) We observed 9 clusters, which included 32 isolates. Altogether cluster isolates represented 36% (32) of the isolates (Table 4); unique genotypes were observed for 50 isolates. The largest cluster was genotype G20, containing the single band 300 bp, followed by cluster genotypes G3 and G23. The genotypes were assigned to 7 genotype groups (I–VII) depending on the total number of different bands observed (Table 3). Most cluster isolates belonged to genotype group I, and all to I and II. A gradual decrease was observed in the number of isolates with increasing number of bands (Figure 1).
Correlations
Correlations were investigated between patient’s sex, age and region, the genotypes, and the resistance pattern.
Correlations related mainly to sex
The general ratio of males to females was 2.5 (63/25) considering all isolates (Table 1). For the Aleppo region, the ratio was 1.4 (21/15), while the M:F ratio for the rest of the regions taken together was 4.2 (42/10). The M:F ratio of cluster isolates was 5.4 (27/5) (Table 3). The M:F ratio for resistant strains in clusters was 4.6 (23/5), while the M:F ratio for the unique genotypes (all were resistant isolates) was 1.9 (33/17) (Table 2). The general M:F ratio for MDR isolates was 2.6 (40/15) (Table 2), but the ratio was 17 (17/1) for MDR isolates in clusters (OR = 6.8, 95% CI: 0.67–70.1, P = 0. 1) (Table 2). Most noticeable was the clearly deviating M:F ratio for MDR in Aleppo, 0.8 (9/11) (OR = 0.27, 95% CI: 0.065–1.14, P = 0.075) (Table 2).
The diversity ratio (i.e. number of genotypes/number of isolates, excluding isolates with no genotype pattern detected, 3 in males and 3 in females) for isolates from males was 0.7 (42/60) and this was clearly different from the diversity ratio for isolates from females which was 1 (22/22). The disproportionately high number of affected males was obvious for most but not all genotype groups: The ratio was 9 (27/3) for the genotype group I (OR = 5.7, 95% CI: 1.5–21.4, P = 0.01), while genotype groups VI and VII included only females (OR = 0.09, 95% CI: 0.009–0.818, P = 0.023) (Table 5). Correlations related mainly to age
The mean age of patients for cluster isolates was 36.6 (SD 4.6) years (median 32, range 17–64 years) and that for cluster genotype G20 was 38.7 (SD 7.9) years (median 32, range 25–64 years); for MDR isolates it was 37.1 (SD 3.4) years (median 35, range 17–65 years) and for MDR cluster isolates 39.1 (SD 6.1) years (median 35, range 17–64 years).
Correlations related mainly to genotype and resistance
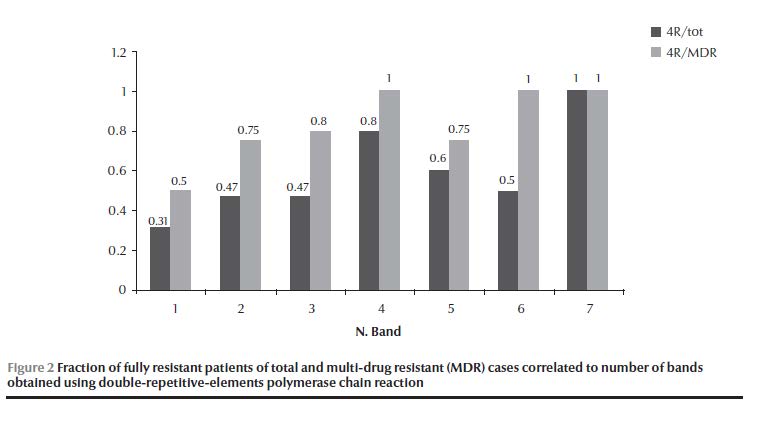
The largest cluster genotype G20 (11 isolates) had 6 isolates that shared a resistance pattern. The rest had each a unique resistance pattern; genotype G23 had 2 out of 4 and genotype G3 had no islolates that shared the same resistance pattern (Table 1). Genotype groups I, II and III had a combined ratio for MDR strains of 0.59 (40/68), while genotype groups IV, V, VI and VII had a combined ratio of 0.79 (11/14) (OR = 2.6, 95% CI: 0.66–10.1, P = 0.17) (Table 4). The percentage of MDR strains was 63% (55/88), higher in unique genotypes (74%, 37/50) than in cluster genotypes (56%, 18/32). For genotype G20 it was 73% (8/11) (OR = 2.1, 95% CI: 0.46–9.29, P = 0.348). Starting with genotype group IV and going to higher genotype group band numbers, all showed 100% STR resistance (Table 6). The STR resistance was most prevalent in unique isolates (94%, 47/50) (OR = 10.1, 95% CI: 2.5–40.7, P < 0.002). However, in cluster isolates, resistance to INH and not to STR was the most common pattern (OR = 1.56, 95% CI: 0.73–3.34, P = 0.25). The fraction of isolates that exhibited the full resistant pattern (RRRR) in the total resistant and in the MDR isolates tended to increase with increasing band number (rs = 0.949, P = 0.026, τ = 0.913, P = 0.035), and this was especially true if only the genotype groups (I, II, III, IV) that included the bulk of isolates (90% of total resistant, 88% of MDR) were considered (rs = 1, P < 0.001, τ = 1,P = 0.021) (Figure 2).
Discussion
The Syrian Arab Republic is considered an endemic country for TB, with an intermediate burden of disease, an estimated incidence for all cases in 2006 of 32 per 100 000 population and a TB prevalence of 40 per 100 000 population [13]. However, the geographic distribution of the disease varies depending on the region. No previous studies in the country have been published using molecular epidemiological techniques applied to M. tuberculosis TB. The current study demonstrates that various genotypes tended to be more common in specific regions than others. The selective distribution of genotype groups, in addition to the observed high level of variability, indicated some level of geographic isolation, and hence separate evolution. It justifies also the initial assignment of the genotypes to major groups according to the general band pattern.
All regions of the country showed high levels of genotype diversity, which is consistent with a low level of transmission of strains in previously treated patients. However, this requires confirmation using a larger pool of patients that would include new cases of TB infection. Not a single case of previously treated TB was observed in which a match in genotype, resistance pattern and location was present. The diversity ratio of 1 for isolates from females and < 1 for isolates from males, if confirmed, might suggest that females have a tendency not to transmit the disease among their sex, but instead infect and are infected by males. One major drawback of using DRE-PCR is the absence of a worldwide accessible databank, mainly due to difficulties of digitizing the results and lack of optimization. Associating obtained patterns with that of digitized genotypes would make future global comparisons of strains possible.
The disproportionately high resistance in isolates of previously treated patients from the Aleppo region should be a cause for further investigation into local treatment procedures, as well as local strain characteristics, given that 69% of these strains were unique to this region and that there were some indications of deviating characteristics. Furthermore, the generally observed prevalence of STR resistance in Syrian patients suggests a need to reconsider the inclusion of STR in current first-line drug treatment.
The well-documented sex disparity for reported TB has been attributed by some authors to socioeconomic and cultural barriers in access to health care [14], and by others to biological and epidemiological characteristics [15,16]. Some studies were able to detect variations in sex distribution depending on disease category [17,18]. The overall reported ratio of males to females in the Syrian Arab Republic was 1.8 according to the World Health Organization country TB profile of 2006 [13]. In the current study the higher ratio of males to females was similar whether isolates in general were considered or only resistant ones or only MDR cases. However, the ratio for clustered genotypes was shifted towards males for unique genotypes, and the ratio was highly shifted towards males when MDR cases in clusters were considered. In this pool of patients males appeared more likely to be infected by MDR strains and to develop MDR, and that was true for specific strains. Females of the same pool were more likely to be affected by MDR in the presence of strains with a higher number of bands (e.g. higher designated numbers of genotype groups). The overall sex ratio of 2.5 (males to females)—which seems to reflect a host sex preference of MTB strains—was in fact an average of dissimilar ratios that appeared to depend heavily on the genotype of the MTB strains.
Resistance to all 4 drugs (RRRR), was the most common pattern in this pool of previously treated patients. The resistance pattern was generally dominated by STR resistance, with EMB resistance being the least observed; however this was not the case in cluster isolates, in which INH resistance became the dominant form of resistance, whereas RIF and EMB resistance rates did not alter. Association of INH resistance with clustering may be explained by the fact that katG 315 mutations are less likely to attenuate bacterial virulence than mutations causing STR resistance [19].
The resistance patterns SSSR, RSSR and RSRR were absent. It is noticeable that these all include resistance to EMB and sensitivity to INH. Considering all observed resistance patterns, resistance to EMB was associated with resistance to INH (and not vice versa). The patterns of concurrent resistance to EMB and INH (4 patterns) involve 51 isolates. The only exception was the pattern SSRR, involving only 1 isolate, which was probably caused by a different mechanism of resistance to EMB. Noticeable also was the fact that all absent resistance patterns (3 patterns) represented EMB resistance and sensitivity to INH. These observations are consistent with both drugs affecting the constitution of the cell wall and with the reported pleiotropic effect of EMB resistance, which causes resistance to other drugs such as INH [20,21]. EMB and INH are also reported to be targets of an efflux pump which confers tolerance to both drugs [22].
The assigned genotype groups appeared to correlate with certain patterns of drug resistance. We observed an increase in the relative occurrence of the total resistance pattern RRRR, being low in the genotype group I and high in genotype group VII. MDR was generally more likely to occur when there were a higher number of genotype bands. The underlying genetic cause of these groupings, and shifts towards specific genotypic groups, could be a consequence of the possible increase in the number of IS6110 elements; such an increase has been implicated or suspected in genotype instability associated with drug resistance and adaptation [23,24]. Moreover, the IS6110 sequence has been shown to possess a 3ʹ outward directed promoter (OP6110) that is able to upregulate downstream genes [25,26]. Given the importance assigned to the PGRS locus and other nearby genes in the context of virulence [27], multiple adjacency of IS6110, or the increased likelihood of correct positioning in the presence of multiple adjacency, may relate to some of the relevant phenotypic correlations observed and is consistent with the recent importance assigned to strain variations in TB [28,29].
Conclusions
The genotyping of M. tuberculosis TB strains, using a slightly modified DRE-PCR, resulted in a Hunter–Gaston discrimination index of D = 0.98. The study of strain variations within this pool of previously treated patients found
a degree of isolation between M. tuberculosis TB strains in different regions of the Syrian Arab Republic, suggesting evolution of significant strain variations;
a gender disparity in the M. tuberculosis transmission dynamic;
distinctive phenotype characteristics of genotype groups assigned based on the number of obtained bands;
phenotypic relevance of the adjacency of mobile elements IS6110 and PGRS;
a correlation between female susceptibility to MDR strains and molecular strain characteristics.
The distinction of strains based on loci that contribute to virulence is important for determining the potential causes of disparities in TB incidence and resistance. Routine, large-scale molecular studies are necessary for investigating the transmission dynamics and mechanisms of disease in the Syrian Arab Republic and neighbouring countries. Relating various strains to strains obtained in other countries using different genotyping procedures is important.
Acknowledgements
We would like to thank the reviewers for their thoughtful comments and suggestions. We acknowledge the financial support of the Ministry of Higher Education, represented by the High Council of Science and the National Commission for Biotechnology in the Syrian Arab Republic. We appreciate the cooperation of the Ministry of Health and Professor Fawaz al Azmeh, head of the National Commission for Biotechnology. We also thank Mrs Mayssoun Elwi, Mrs Nibal Qirahkahya and Ms Buthaina Salamah for their efforts.
References
- Tanaka MM. Evidence for positive selection on Mycobacterium tuberculosis within patients. BMC Evolutionary Biology, 2004, 4:31 (doi: 10.1186/1471-2148-4-3).
- Zigol M et al. Patients with previously treated tuberculosis no longer neglected. Clinical Infectious Diseases, 2007, 44:61–64.
- Verver S et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. American Journal of Respiratory and Critical Care Medicine, 2005, 171:1430–1435.
- Martínez M et al. Impact of laboratory cross-contamination on molecular epidemiology studies of tuberculosis. Journal of Clinical Microbiology, 2006, 44(8):2967–2969.
- European Concerted Action on New Generation Genetic Markers and Techniques for the Epidemiology and Control of Tuberculosis. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerging Infectious Diseases, 2006, 12(5):736–743.
- Montoro E et al. Molecular fingerprinting of Mycobacterium tuberculosis isolates obtained in Havana, Cuba, by IS6110 restriction fragment length polymorphism analysis and by the double-repetitive-element PCR method. Journal of Clinical Microbiology, 1998, 36:3099–3102.
- Sola C et al. Spoligotyping followed by double-repetitive-element PCR as a rapid alternative to IS6110 fingerprinting for epidemiological studies in tuberculosis. Journal of Clinical Microbiology, 1998, 36:1122–1124.
- Kubica GP et al. Sputum digestion and decontamination with N-acetyl-L-cysteine-sodium hydroxide for culture of mycobacteria. American Review of Respiratory Disease, 1963, 78:775–779.
- Antimycobacterial susceptibility testing for Mycobacterium tuberculosis. Tentative standard M24-T. Villanova, Pennsylvania, National Committee for Clinical Laboratory Standards, 2002.
- Harris E. A low-cost approach to PCR, appropriate transfer of biomolecular techniques. Oxford, Oxford University Press, 1998.
- Friedman CD et al. Double-repetitive-element PCR method for subtyping Mycobacterium tuberculosis clinical isolates. Journal of Clinical Microbiology, 1995, 33:1383–1384.
- Hunter PR, Gaston MA. Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. Journal of Clinical Microbiology, 1998, 26:2465–2466.
- Countries. World Health Organization [website] (http://www.who.int/countries/en/, accessed 5 January 2010).
- Karim F et al. Gender differences in delays in diagnosis and treatment of tuberculosis. Health Policy and Planning, 2007, 22(5):329–324.
- Pérez-Guzmán C et al. Diabetes modifies the male:female ratio in pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease, 2003, 7(4):354–358.
- Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression of Toll-like receptor 4 in the mouse, a trigger for inflammation and innate immunity. Biology of Reproduction, 2008, 78(3):432–437.
- Razanamparany VR et al. Extrapulmonary and pulmonary tuberculosis in Antananarivo (Madagascar): high clustering rate in female patients. Journal of Clinical Microbiology, 2002, 40(11):3964–3969.
- Tuberculosis in Australia: bacteriologically confirmed cases and drug resistance, 1998–1999: report of the Australian Mycobacterium Reference Laboratory Network. Communicable Diseases Intelligence, 2001, 25(4):261–265.
- Pym AS, Saint-Joanis B, Cole ST. Effect of katG mutations on the virulence of Mycobacterium tuberculosis and the implication for transmission in humans. Infection and Immunity, 2002, 70(9):4955–4960.
- Shen X et al. Association between embB codon 306 mutations and drug resistance in Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy, 2007, 51(7):2618–2620.
- Hazbo’n MH et al. Role of embB Codon 306 mutations in Mycobacterium tuberculosis revisited: a novel association with broad drug resistance and IS6110 clustering rather than ethambutol resistance. Antimicrobial Agents and Chemotherapy, 2005, 49(9):3794–3802.
- Colangeli, R et al. The Mycobacterium tuberculosis iniA gene is essential for activity of an efflux pump that confers drug tolerance to both isoniazid and ethambutol. Molecular Microbiology, 2005, 55(6):1829–1840.
- Tanaka MM, Rosenberg NA, Small PM. The control of copy number of IS6110 in Mycobacterium tuberculosis. Molecular Biology and Evolution, 2004, 21:2195–2201.
- Farnia P et al. Instability of IS6110 patterns in multidrug-resistant strains of Mycobacterium tuberculosis. Epidemiology and Infection, 2007, 135:346–352.
- Beggs ML, Eisenach KD, Cave MD. Mapping of IS6110 insertion sites in two epidemic strains of Mycobacterium tuberculosis. Journal of Clinical Microbiology, 2000, 38(8):2923–2928.
- Safi H et al. IS6110 functions as a mobile, monocyte-activated promoter in Mycobacterium tuberculosis. Molecular Microbiology, 2004, 52(4):999–1012.
- Gey van Pittius NC et al. Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evolutionary Biology, 2006, 6:95 (doi: 10.1186/1471-2148-6-95).
- Gillespie SH. Evolution of drug resistance in Mycobacterium tuberculosis: clinical and molecular perspective. Antimicrobial Agents and Chemotherapy, 2002, 46(2):267–274.
- Hanekom M et al. A recently evolved sublineage of the Mycobacterium tuberculosis Beijing strain family is associated with an increased ability to spread and cause disease. Journal of Clinical Microbiology, 2007, 45(5):1483–1490.