A. Ajem,1 A. Slama,2 F.B.H. Slama1 and T. Mehjoub3
معدل انتشار طفرة ليدن في العامل الخامس بين مرضى الخُثَار في تونس
عايدة عجم، عمر سلامة، فؤاد بن الحاج سلامة، تهامي محجوب
الخلاصـة: يتعرف الباحثون في هذه الدراسة على معدل انتشار طفرة ليدن الموروثة في العامل الخامس ضمن مجموعة من مرضى الـخُثَار تعدادهم 128 مريضاً، (102 مريضاً منهم لديه خُثار وريدي و26 منهم لديه خُثار شرياني)، وهم من المترددين على إحدى مستشفيات سوسة في تونس، إلى جانب مجموعة شواهد تعدادهم مئة ليس لديهم سوابق خُثار. وقد وجد الباحثون طفرة ليدن في العامل الخامس باستخدام تقنية التضخيم بالتفاعل السلسلي للبوليميراز النوعية للأليل لدى عدد أكبر بقدر يعتد به إحصائياً من المرضى (20.3) مما لدى الشواهد (%6.0). وقد كان معدل الانتشار الأكثر ارتفاعاً ذا اعتداد إحصائي في مجموعة فرعية من مرضى الـخُثَار الوريدي وليس في مرضى الـخُثَار الشرياني. وقد كان تكرار الأليل %3.5 بين السكان التونسيين الأسوياء، إن تحري المرضى التونسيين المصابين بالـخُثَار الوريدي وأقربائهم لكشف طفرة ليدن في العامل الخامس أمر يمكن تبريره.
ABSTRACT This study determined the prevalence of inherited factor V Leiden mutation in a group of 128 thrombosis patients (102 with venous thrombosis and 26 with arterial thrombosis) attending a hospital in Sousse, Tunisia, and a control group of 100 with no history of thrombosis. Using an allele-specific PCR amplification technique, factor V Leiden was found in significantly more patients (20.3%) than controls (6.0%). The higher prevalence was significant in the subgroup of venous thrombosis patients but not in arterial thrombosis patients. The allele frequency was 3.5% in the normal Tunisian population. Screening Tunisian patients with venous thrombosis and their relatives for factor V Leiden may be justified.
Prévalence de la mutation Leiden du facteur V chez des sujets atteints de thrombose en Tunisie
RÉSUMÉ Cette étude a déterminé la prévalence de la mutation Leiden du facteur V héréditaire dans un groupe de 128 sujets atteints de thrombose (102 de thrombose veineuse et 26 de thrombose artérielle) fréquentant un hôpital de Sousse (Tunisie) et dans un groupe témoin de 100 sujets sans antécédents thrombotiques. Grâce à une technique d’amplification par PCR spécifique de l’allèle, le facteur V Leiden a été détecté chez un nombre de patients significativement plus élevé (20,3 %) que de témoins (6,0 %). La prévalence supérieure était significative dans le sous-groupe des patients présentant une thrombose veineuse mais pas dans celui des patients présentant une thrombose artérielle. La fréquence de l’allèle était de 3,5 % dans la population tunisienne normale. Le dépistage du facteur V Leiden chez les patients tunisiens atteints de thrombose veineuse et les membres de leur famille peut se justifier.
1Immunogenetic Unit, Faculty of Medicine of Sousse, Sousse, Tunisia (Correspondence to F.B.H. Slama:
2Department of Anaesthesia and Intensive Care Medicine; 3Laboratory of Haematology, Farhat Hached Hospital, Sousse, Tunisia.
Received: 12/12/06; accepted: 14/06/07.
EMHJ, 2009, 15(6):1483-1488
Introduction
Disorders of the haemostatic mechanisms that contribute towards a predisposition to thrombosis (thrombophilia) may be a consequence of both acquired and inherited or genetic causes. Interest in the genetic basis of thrombosis was accelerated with the discovery of the factor V Leiden (FVL) mutation, which is considered the most common genetic risk factor [1]. FVL is characterized by a single adenine (A) for guanine (G) point mutation at nucleotide 1691 in the gene coding for coagulation factor V. Factor V is a single-chain pro-cofactor that acts in concert with other plasma factors in regulating blood coagulation [2]. FVL is inactivated 10–20 times more slowly than the native form of factor V, leading to excessive thrombin generation and a presumed lifelong prothrombotic tendency [3]. Carriers of the factor V 1691A allele have been shown to have an increased risk for venous thrombosis.
FVL occurs in 20% of patients with deep vein thrombosis compared with 5% in the normal population [1]. Whereas many studies reported FVL as an inherited predisposing factor for venous thrombosis, its association with arterial thrombosis is less clear-cut [4–6]. However, a link between poor obstetric outcome, including pregnancy loss, and FVL has been established [7,8]. Studies on the prevalence of the FVL mutation have revealed an uneven ethnic and geographical distribution. It is relatively frequent in normal individuals in populations of European origin (mean allelic frequency 2.7%), while it is virtually absent in natives of Africa, America, Asia and Australia [9,10]. Furthermore, in Europe an increasing cline of FVL prevalence is observed from west to east [9].
In view of the lack of data in the Tunisian population on the role of FVL in thrombotic disease, we assessed the prevalence of FVL mutation among thrombosis patients and healthy subjects in a hospital in Sousse, Tunisia.
Methods
Subjects
From 2002 to 2004, we investigated 128 patients with a personal history of thrombosis recruited consecutively from those attending Farhat Hached Hospital, Sousse. The mean age of patients was 37 [standard deviation (SD 8)] years, range 1–72 years. Among these patients, 102 had suffered venous thrombosis and 26 arterial thrombosis.
A control group of 100 healthy volunteers, mean age 31 (SD 9) years, range 15–61 years, were randomly recruited from blood bank donors. The subjects were unrelated to the patients and were taken to represent the general Tunisian population. None of the healthy subjects reported any past history of thrombosis, heart problems or a family history of thrombotic disease.
The study was conducted after all institutional ethics requirements were met.
Data collection
EDTA-anticoagulant blood (5 mL sample) was obtained from each participant and processed shortly afterwards.
Using a proteinase K and a saline extraction protocol, genomic DNA was extracted from EDTA anticoagulant blood according to Miller et al. [11]. Briefly, buffy coats of nucleated cells were resuspended with 3 mL nuclear lysis buffer (10 mM tris-HCl, 400 mM NaCl and 2 mM EDTA, pH 8.2). The cell lysates were digested overnight at 37 °C with a proteinase K solution (Sigma, Germany) (1 mg proteinase K, 3% sodium dodecyl sulfate and 2 mM EDTA). After digestion, proteins precipitated by 1 mL of 6 M NaCl and 2 volumes of absolute ethanol were added to the supernatant. The precipitated DNA was dissolved in 200 μL tris-EDTA buffer (10 mM tris-HCl, 0.2 mM EDTA, pH 7.5).
The mutation was assessed by an allele-specific amplification polymerase chain reaction (ASA-PCR) technique [12], which requires 2 different PCR reactions (2 tubes). The reactions were carried out using the common primer 5′-TGTTATCACACTGGTGCTAA-3′ (127 to 146 in intron 10) and an allele-specific primer differing by the 3′ OH extremity corresponding to the nucleotide 1691 (in exon 10) either specific of the mutated allele (A) 5′-CAGATCCCTGGACAGACA-3′ or specific of the wild allele (G) 5′-CAGATCCCTGGACAGACG-3′. PCR amplification generated a 174 bp fragment.
To validate the negative results, coagulation factor IX was coamplified as a PCR control in the ASA PCR reaction and generated a 250 bp fragment. We used the primers described by Reitsma et al. [13].
The PCR mixture contained 200 ng genomic DNA, 20 pmol each oligonucleotide (Biogene, Tunisia), 200 μM each dNTP (Pharmacia, France), 1 × PCR buffer (10 mM tris-HCl, 50 mM KCl, 0.1%
Triton® X-100), 25 mM MgCl2 and 1 unit of taq-polymerase (Promega, France) in a final volume of 50 µL. Reactions were incubated in a DNA thermal cycler (Touchgene gradient). The thermal profile consisted of 4 min denaturation at 94 °C, followed by 32 cycles consisting of 40 s denaturation at 94 °C, 40 s annealing at 56 °C, and 40 s extension at 72 °C. Samples were then maintained at 72 °C for 7 min.
Separation of the DNA fragments was achieved using a conventional horizontal gel electrophoresis apparatus (Biorad, France): 10 µL of the amplified products were electrophoresed on a 1.5% agarose gel in the presence of tris-borate-EDTA buffer (100 mM tris-borate, 2 mM EDTA) and visualized by ethidium bromide staining (2 μg/mL) incorporated in the matrix.
Statistical analysis
Allelic frequencies were calculated by the gene-counting method. The chi-squared test was used to ascertain whether genotype distributions were in agreement with those expected by the Hardy–Weinberg equilibrium. The statistical significance of differences in carrier and allele frequencies for FVL between the study groups was determined using the chi-squared test and the Yates corrected chi-squared test. Values below 0.05 were considered significant.
Results
The presence of FVL was investigated in 128 thrombosis patients and 100 healthy subjects. The determination of the normal or mutant genotype is based on the presence or absence of the corresponding fragment (Figure 1).
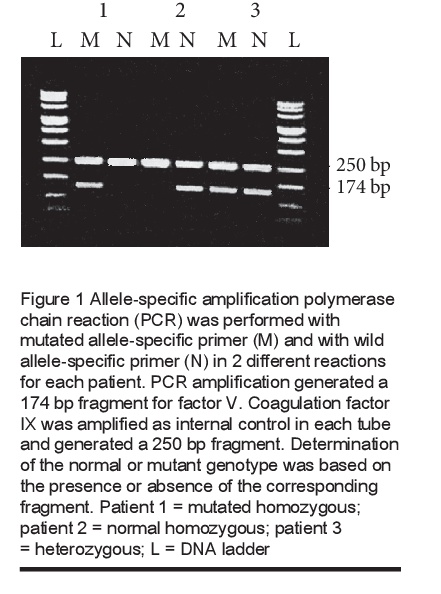
FVL was found in 26 patients and in 6 healthy controls. The genotype frequencies are given in Table 1. The difference in the prevalence of FVL between the 2 groups was statistically significant (P < 0.01).
Of the 6 healthy subjects who tested positive for FVL, 5 were heterozygous (G/A), giving an overall allele frequency of 3.5% in the normal population. The genotype distribution in healthy individuals was in accordance with the Hardy–Weinberg equilibrium using the chi-squared test (P > 0.05). Of the 26 patients who tested positive for FVL, 16 were heterozygous, giving an allele frequency of 14.1% in the thrombosis population.
Of the 26 arterial thrombosis patients studied, 1 was heterozygous for the FVL mutation, giving a prevalence rate of 3.8% which was not statistically different from that in healthy subjects (6.0%) (P > 0.05). In contrast, FVL prevalence was significantly higher in venous thrombosis patients (24.5%) compared with healthy subjects (P < 0.01). Allele frequencies are given in Table 1.
Discussion
In order to determine the relation between FVL and thrombotic disorders, we assessed the prevalence of this mutation among Tunisian thrombosis patients and a healthy control group. FVL was found in 25 out of 102 (24.5%) venous thrombosis patients compared with 6 out of 100 (6.0%) healthy subjects.
While our results were in agreement with previously published data documenting the increased prevalence of FVL mutation among venous thrombosis patients [5,14–16], they were in apparent contradiction with others that failed to establish a link between FVL and venous thrombosis [17,18]. This apparent contradiction may be due to the very low prevalence of FVL among healthy subjects in these studies.
The contribution of FVL to the risk of venous thrombosis seems to be higher in patients from areas where the prevalence of FVL mutation is high among the normal population. These data suggest that ethnic considerations are important when testing for FV-Leiden. In this study, the prevalence of the FVL mutation was high in the normal Tunisian population (3.5%). While the number of subjects was rather small, our data are in agreement with previous findings that showed a high prevalence of FVL among Tunisians [19] compared with other Arab populations [20].
The finding of a very different geographical distribution of FVL could possibly explain, at least in part, the difference in the prevalence of venous thrombosis in different parts of the world. In areas with high FVL prevalence, the relative risk of thrombosis in FVL heterozygotes is 3–8 times higher than in the general population, whereas the increased risk of thrombosis in homozygotes is estimated to be 50–80-fold greater than those without the defect [21]. Hence it is important to identify these high-risk patients to provide adequate counselling about the risk of thrombosis before elective surgical procedures and before taking birth control pills or hormonal replacement. Moreover, the presence of FVL should increase the optimal treatment duration after a first thrombotic event [22]. There are no studies that give clear guidance on the optimum management of clinically unaffected carriers.
In contrast with the results in venous thrombosis patients, the rate of FVL of in arterial thrombosis patients was not statistically significantly different from the rate for control subjects. Therefore FVL did not seem to play a role in arterial disease. Despite the small number of arterial thrombosis patients in this study, our results were in harmony with other reports [5,6,23]. Nevertheless, additional cofactors such as hyperhomocysteinaemia may predispose carriers of FVL mutation to an increased risk for arterial thrombosis [24]. Further studies are necessary for a better understanding of the interaction between FVL and arterial thrombosis .
In view of the high prevalence of the FVL mutation among Tunisian patients with VT, screening for FVL seems to be justified in these patients. Screening for the FVL mutation may also be recommended in high-risk groups such as relatives of FVL carriers and those with additional risk factors.
References
- Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet, 1999, 353:1167–73.
- Kalafatis M et al. Characterization of the molecular defect in factor V R506Q. Journal of biological chemistry, 1995, 270:4053–7.
- Orban T, Kalafatis M, Gogonea V. Completed three-dimensional model of human coagulation factor Va. Molecular dynamics simulations and structural analysis. Biochemistry, 2005, 44:13082–90.
- Taymaz H et al. Sequence variations within the genes related to hemostatic imbalance and their impact on coronary artery disease in Turkish population. Thrombosis research, 2007, 119:55–62.
- Irani-Hakime N et al. Factor V R506Q mutation-Leiden: an independent risk factor for venous thrombosis but not coronary artery disease. Journal of thrombosis and thrombolysis, 2001, 11:111–6.
- Aleksic M et al. Comparison of the prevalence of APC-resistance in vascular patients and in a normal population cohort in Western Germany. European journal of vascular and endovascular surgery, 2005, 30:160–3.
- Alonso A et al. Acquired and inherited thrombophilia in women with unexplained fetal losses. American journal of obstetrics and gynaecology, 2002, 187:1337–42.
- Onderoglu L et al. High frequency of thrombophilic disorders in women with recurrent fetal miscarriage. Clinical and experimental obstetrics & gynecology, 2006, 33:50–4.
- Lucotte G, Mercier G. Population genetics of factor V Leiden in Europe. Blood cells, molecules and diseases, 2001, 27:362–7.
- Bauduer F, Lacombe D. Factor V Leiden, prothrombin 20210, methylenetetrahydrofolate reductase 677T, and population genetics. Molecular genetics and metabolism, 2005, 86:91–9.
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic acids research, 1988, 16:1215.
- Hezard N et al. Factor V Leiden: detection in whole blood by ASA PCR using an additional mismatch in antepenultimate position. Thrombosis research, 1997, 88:59–66.
- Reitsma PH et al. The putative factor IX gene promoter in hemophilia B Leyden. Blood, 1988, 72:1074–6.
- Cushman M et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. American journal of medicine, 2004, 117:516–22.
- Colaizzo D et al. Gain-of-function gene mutations and venous thromboembolism: distinct roles in different clinical settings. Journal of medical genetics, 2007, 44:412–6.
- Juul K et al. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Annals of internal medicine, 2004, 140:330–7.
- Pathare A et al. Hereditary thrombophilia in ethnic Omani patients. American journal of haematology, 2006, 81:101–6.
- Angchaisuksiri P et al. Prevalence of the G1691A mutation in the factor V gene (factor V Leiden) and the G20210A prothrombin gene mutation in the Thai population. American journal of haematology, 2000, 65:119–22.
- Bouaziz L et al. Allelic frequency of the factor V Leiden mutation and of the prothrombin gene 20210A mutation in healthy Tunisian population. Thrombosis and haemostasis, 2004, 91:824–5.
- Almawi WY et al. Varied prevalence of factor V G1691A (Leiden) and prothrombin G20210A single nucleotide polymorphisms among Arabs. Journal of thrombosis and thrombolysis, 2005, 20:163–8.
- Caprini JA et al. Laboratory markers in the diagnosis of venous thromboembolism. Circulation, 2004, 109:14–8.
- Gallus AS. Management options for thrombophilias. Seminars in thrombosis and hemostasis, 2005, 31:118–26.
- Zee RY et al. Multi-locus candidate gene polymorphisms and risk of myocardial infarction: a population-based, prospective genetic analysis. Journal of thrombosis and haemostasis, 2006, 4:341–8.
- Page C et al. Arterial thrombosis associated with heterozygous factor V Leiden disorder, hyperhomocysteinemia, and peripheral arterial disease: importance of synergistic factors. Journal of vascular surgery, 2005, 42:1014–8.


