A.G.M. Abdel Gader,1 S.H. Abdullah1 and A.Y. Kordofani 2
مستويات الهوموسيستئين في البلازما لدى مرضى القلب والأوعية والملاريا وسوء التغذية بالبروتين والطاقة في السودان
عبد الجليل عبد القادر، سامي عبد الله، أنوار كردفانى
الخلاصـة: يدرس الباحثون في هذه الدراسة دور فرط الهوموسيستئين في الدم باعتباره أحد عوامل الاختطار لدى السودانيين البالغين الذين يعانون من مرض القلب والأوعية أو الملاريا، أو الأطفال المصابين بسوء التغذية بالبروتين والطاقة. وقد وجد الباحثون أن المستويات المتوسطة للهوموسيستئين الكلي في البلازما (مقدرة بمكرومول/لتر) أعلى بمقدار يعتد به إحصائياً لدى المصابين بمرض القلب التاجي (17.64، بانحراف معياري 11.86) وبالخُثار الوريدي المتكرر (15.06، بانحراف معياري 10.55) وبالملاريا المتكررة (13.61، بانحراف معياري 4.82) مما لدى الشواهد الأصحاء (7.85، بانحراف معياري 3.39). كما كانت المستويات المتوسطة للهوموسيستئين أعلى بمقدار يعتد به إحصائياً لدى الأطفال المصابين بسوء التغذية بالبروتين والطاقة (8.41، بانحراف معياري 1.61) مما لدى الأطفال الشواهد الأصحاء (5.72، بانحراف معياري 1.99).
ABSTRACT This study investigated the role of hyperhomocysteinaemia as a risk factor in Sudanese adults suffering from cardiovascular disease or malaria and children with protein–energy malnutrition. Mean total plasma homocysteine levels (µmol/L) were significantly higher in patients with coronary heart disease (17.64; SD 11.68) recurrent venous thrombosis (5.06; SD 10.55) and recurrent malaria (13.61; SD 4.82) than in healthy adult controls (7.85; SD 3.39). The mean homocysteine level was also significantly higher in children with protein–energy malnutrition (8.41; SD 1.61) than in healthy control children (5.72; SD 1.99).
Taux d’homocystine plasmatique dans les maladies cardiovasculaires, le paludisme et la malnutrition protéino-énergétique au Soudan
RÉSUMÉ Cette étude s’est penchée sur le rôle de l’hyperhomocystéinémie en tant que facteur de risque chez des adultes soudanais souffrant de maladie cardiovasculaire ou de paludisme et chez des enfants atteints de malnutrition protéino-énergétique. Les taux moyens d’homocystéine totale plasmatique (µmol/L) étaient significativement plus élevés chez les adultes présentant une coronaropathie [17,64 (E.T. 11,68)], une thrombose veineuse récidivante [15,06 (E.T. 10,55)] et un paludisme récurrent [13,61 (E.T. 4,82)] que chez les adultes témoins sains [7,85 (E.T. 3,39)]. En outre, le taux moyen d’homocystéine était significativement plus élevé chez les enfants atteints de malnutrition protéino-énergétique [8,41 (E.T. 1,61)] que chez les enfants témoins sains [5,72 (E.T. 1,99)].
1Coagulation Laboratory, Department of Physiology, College of Medicine, King Saud University, Riyadh, Saudi Arabia (Correspondence to A.G.M. Abdel Gader:
2Department of Pathology, Faculty of Medicine, University of Khartoum, Khartoum, Sudan.
Received: 10/04/07; accepted: 30/08/07
EMHJ, 2009, 15(6):1432-1439
Introduction
Elevated levels of plasma homocysteine (hyperhomocysteinaemia) have been linked to a variety of disease conditions, particularly coronary artery disease and venous thromboembolism [1–3]. Even where hyperhomocysteinaemia is a well established risk factor, we do not know the exact mechanism whereby high homocysteine levels cause thromboembolic disease [4] and the issue has not been studied in Sudanese patients, where the prevalence of these diseases is assumed to be low.
In tropical developing countries, including Sudan, malaria and malnutrition are still major public health problems and are more prevalent than thromboembolic disease. There are limited data, however, on fluctuations in homocysteine levels in these conditions, particularly malaria [5,6], and in protein–energy malnutrition (PEM) [7] to determine whether hyperhomocysteinaemia is also risk factor in these diseases.
The aim of this study was to investigate the role of hyperhomocysteinaemia as a risk factor in Sudanese adults suffering from coronary heart disease (CHD), recurrent venous thrombosis (VT) or recurrent malaria, and in children suffering from PEM.
Methods
Patient groups
Patients were recruited from the 3 teaching hospitals in the Sudanese triple capital Khartoum, Omdurman and Khartoum North over the period February 2006–March 2007. A total of 146 patients were selected from the outpatient clinics and wards, using the random number procedure where consultants were requested to pick 1 patient for every 3 to 4 attendants at the outpatient clinics or admission to hospital.
Participants were divided into 4 groups:
- Patients with CHD (n = 50): 43 men and 7 women [mean age 55.6 (standard deviation (SD) 14.4 ) years; mean weight 77.3 (SD 8.3) kg]. These patients were diagnosed by history, medical examination, electrocardiogram, echocardiogram and blood tests, including enzymes (troponin T and creatine phosphokinase).
- Patients with recurrent VT (n = 26): 17 men and 9 women [mean age 50.9 (SD 14.7) years; mean weight 73.6 (SD 10.9) kg]. Their diagnosis was based on history, medical examination and Doppler ultrasound imaging.
- Patients with recurrent malaria (n = 50): 32 men and 18 women [mean age 36.8 (SD 10.1) years; mean weight 74.9 (SD 9.7) kg]. All patients were confirmed with Plasmodium falciparum infection using the thick-blood film technique. Recurrence referred to any recurrence of symptoms of malaria (in addition to positive thick-blood film test) after a previous malarial attack.
- Children with PEM (n = 20): 13 boys and 7 girls [mean age 1.3 (SD 0.7) years; mean weight 6.5 (SD 1.9) kg]. Malnutrition was diagnosed according to Wellcome classification of malnutrition criteria [8].
Consent was taken from adult subjects and the guardians of children before blood sampling.
Healthy subjects
Healthy Sudanese controls were recruited from copatients, colleagues and blood donors and children coming for vaccination or routine health checks.
- Adult controls: 75 men [mean age 45.2 (SD 13.2) years; mean weight 82.0 (SD 10.8) kg] and 75 women [mean age 37.7 (SD 11.1) years; mean weight 71.1 (SD 12.6) kg].
- Child controls: 25 boys [mean age 7.4 (SD 2.8) years; mean weight 24.7 (SD 7.2) kg] and 25 girls [mean age 7.6 (SD 3.2) years; mean weight 26.1 (SD 6.5) kg].
Blood collection and processing
A 2.5 mL blood sample was collected from each participant by venepuncture using a plastic disposable syringe (Mediject, South Korea) into tubes containing EDTA after an overnight fast (EDTA plasma is recommended in the assay used in this study). As homocysteine is synthesized by erythrocytes and leukocytes and continues to be produced following collection of blood, the sample tubes were placed on ice and the plasma was separated from blood cells within 30 minutes. For the children 2.5 mL blood were added to plain plastic tubes and serum was separated and used for the assay of vitamin B12 and folic acid.
Laboratory methods
Homocysteine measurement was done by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Homocysteine Microtiter Plate Assay, Diazyme, Poway, California) and the plates were read in the Stat Fax 2100 Microplate Reader (Awareness Technology, Palm City, Florida). Vitamin B12 and folic acid measurements were done with a chemiluminescence technique with reagents supplied by Bayer HealthCare (Diagnostics Division, Torrytown, New York).
Statistical methods
SPSS, version 11 was used for data analysis. The data were expressed as mean and SD. Student t-test for independent groups was employed in the comparison between the healthy controls and patient groups. One-way analysis of variance (ANOVA) was used to compare all parameters for different group of patients and multiple comparisons were used to find the significance between case and control groups. Correlation coefficients were calculated, where appropriate, between the different parameters for normal and abnormal groups.
Results
The mean total plasma homocysteine level was 7.85 (SD 3.39) µmol/L, and was significantly higher in males [8.42 (SD 4.09) µmol/L] than females [7.27 (SD 2.40) µmol/L] (P < 0.05).
The mean total plasma homocysteine level was significantly higher in patients with CHD [17.64 (SD 11.68) µmol/L], recurrent VT [15.06 (SD 10.55) µmol/L] and recurrent malaria [13.61 (SD 4.82) µmol/L] than in healthy adult controls [7.85 (SD 3.39) µmol/L] (P < 0.0001) (Table 1).
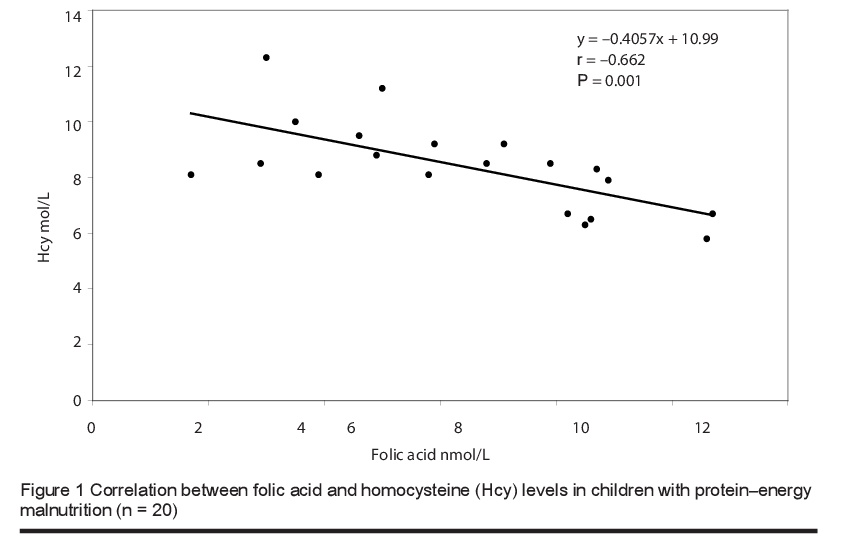
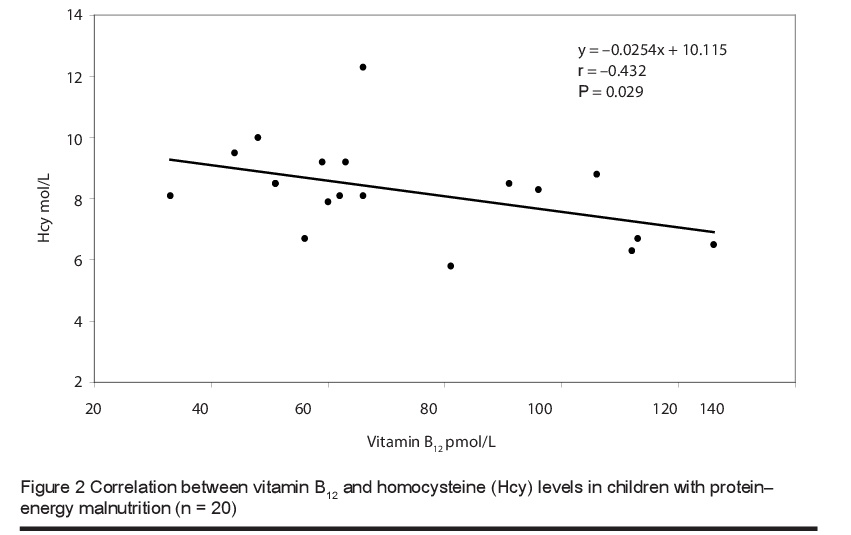
The mean level of total plasma homocysteine was significantly higher in children with PEM [8.41 (SD 1.61) µmol/L] than in healthy children [5.71 (SD 1.99) µmol/L] (P < 0.0001). The vitamin B12 levels were 72.55 (SD 26.13) versus 290.5 (SD 115.66) pmol/L respectively and for folic acid were 6.36 (SD 2.63) versus 13.38 (SD 2.67) nmol/L (P < 0.0001) (Table 2). Furthermore, there was a significant negative correlation between the blood levels of total homocysteine and folic acid (Figure 1) and total homocysteine and vitamin B12 (Figure 2) in children with PEM.
Discussion
Many studies have confirmed that hyperhomocysteinaemia plays a major role in the pathogenesis of cardiovascular disease and other conditions characterized by disturbed haemostasis [1–3]. However, very few studies in this area emerged from underdeveloped countries, where the prevalence of cardiovascular diseases is lower than in developed countries [9] and it is not known whether hyperhomocysteinaemia plays any pathognomic role in cardiovascular and other more prevalent diseases in the tropics. The current study, which we believe is the first of its type in Sudanese patients, therefore offers a unique chance to explore the involvement of hyperhomocysteinaemia in cardiovascular disease as well as malaria and PEM.
Blood samples were collected from healthy controls and patients with CHD, recurrent VT and recurrent malaria and from children with PEM, who usually suffer from multiple vitamin deficiencies [10]. The levels of homocysteine were measured by ELISA, which correlates well with the widely used high-pressure liquid chromatography method [11].
Our finding of significantly higher total plasma homocysteine concentrations in adult males than in females is in line with many earlier studies in different populations and ethnic groups [12–16]. Although earlier studies found higher homocysteine levels in boys than in girls [17,18], in our study the difference was not statistically significant.
Total plasma homocysteine levels in the CHD patients were significantly higher than in healthy controls. These findings are in line with similar studies in industrialized countries [1,2,19]. There is no agreement on the pathophysiological mechanism
underlying the association between hyperhomocysteinaemia and clot formation in thromboembolic diseases. While some studies found a significant procoagulant effect of homocysteine on endothelial cells [20], others could not find evidence of haemostatic activation in hyperhomocysteinaemia [18].
Hyperhomocysteinaemia is known to be more prevalent in patients with VT than in healthy subjects [3]. Our findings agree with this observation; the mean level of total plasma homocysteine was significantly higher in patients with recurrent VT than in healthy controls, indicating that hyper-homocysteinaemia is a risk factor for arterial and VT disease in our patients.
Very few studies have probed the relationship between homocysteine and malaria infection and this could be due to the geographical distribution of malaria, which is a disease of tropical countries where local researchers have not taken an active interest in this subject. We found significantly higher levels of total plasma homocysteine in patients with recurrent malaria than in healthy subjects. Our finding gives support to the recent report of Chillemi et al., who further confirmed that homocysteine levels correlated positively with severity of the disease as reflected by the degree of parasitaemia [5].
The pathogenic relationship between hyperhomocysteinaemia and malaria is open to speculation. It is possible that the presence of S-adenosylhomocysteine hydrolase in the P. falciparum parasites results in the accumulation of homocysteine in plasma [6]. P. falciparum infection is known to be associated with disturbance of the folate-vitamin B12-methionine metabolic pathway and/or haemolysis, leading to relative deficiencies of B12 and folic acid, which in turn result in elevation of homocysteine levels [21].
In the current study we found significantly lower levels of serum folic acid and B12 and higher levels of plasma homocysteine in children with PEM than in healthy controls. There was also significant negative correlation between the levels of homocysteine and the levels of these vitamins, and this is in line with earlier studies [15,17,18]. The close association between the levels of these 2 vitamins and homocysteine levels was confirmed in a study in Taiwan that focused on the effect of a vegetarian diet on B-vitamin status and plasma homocysteine level [22]. Significantly higher levels of plasma folate were found in vegetarians compared to omnivores, but lower levels of vitamin B12. Fasting plasma homocysteine levels were significantly higher in vegetarians than in omnivores [11.20 (SD 4.27) versus 8.64 (SD 2.06) µmol/L; median: 10.5 versus 8.5 µmol/L]. In agreement with our study, fasting plasma homocysteine correlated inversely with plasma folate and vitamin B12 levels in the vegetarian group. This negative correlation was also reported in studies performed among children in Brazil [23] and Guatemala [24].
The occurrence of hyperhomocysteinaemia in patients with PEM has also been ascribed to the shrinking of endogenous nitrogen pools as a result of decreased protein intake [7] and accordingly, raised total homocysteine may be an attempt by the malnourished and/or stressed body to preserve methionine homeostasis [7]; the authors further suggested that low concentrations of folic acid and vitamin B12 may play a role in the elevation of plasma homocysteine levels.
In conclusion, the current study on homo-cysteine levels in health and disease in Sudanese subjects revealed that, as in reports from developed countries, hyperhomocysteinaemia is associated with CHD and VT. In addition, hyperhomocysteinaemia features significantly in patients with recurrent malaria and in children with PEM. Thus hyperhomocysteinemia seems to underlie the aetiopathogenesis of diverse disease conditions, with no clear geographical or ethnic boundaries.
References
- Alfthan G et al. Association between serum homocysteine and coronary heart disease in a Finnish high-risk male population. Atherosclerosis supplements, 2002, 3(2):66.
- Ogawa M et al. Homocysteine and hemostatic disorders as a risk factor for early myocardial infarction at young age. Thrombosis research, 2003, 109(5–6):253–8.
- Hainaut P et al. Hyperhomocysteinemia and venous thromboembolism: a risk factor more prevalent in the elderly and in idiopathic cases. Thrombosis research, 2002, 106(2):121–5.
- Herrmann W, Herrmann M, Obeid R. Hyperhomocysteinaemia: a critical review of old and new aspects. Current drug metabolism, 2007, 8(1):17–31.
- Chillemi R et al. Hyperhomocysteinemia in acute Plasmodium falciparum malaria: an effect of host–parasite interaction. Clinica chimica acta, 2004, 348:113–20.
- Tanaka N et al. Crystal structure of s-adenosyl-l-homocysteine hydrolase from the human malaria parasite Plasmodium falciparum. Journal of molecular biology, 2004, 343:1007–17.
- Ingenbleek Y, Hardillier E, Jung L. Subclinical protein malnutrition is a determinant of hyperhomocysteinemia. Nutrition, 2002, 18:40–6.
- Wellcome Trust Working Party. Classification of infantile malnutrition. Lancet, 1970, 2:302–3.
- Lopez AD, Mathers CD. Measuring the global burden of disease and epidemiological transitions: 2002–2030. Annals of tropical medicine and parasitology, 2006, 100(5–6):481–99.
- Singh MB et al. Studies on the nutritional status of children aged 0–5 years in a drought-affected desert area of western Rajasthan, India. Public health nutrition, 2006, 9(8):961–7.
- Ubbink JB, Vermaak WJH, Bissbort S. Rapid high-performance liquid chromatographic assay for total homocysteine levels in human serum. Journal of chromatography, 1991, 565(1–2):441–6.
- Golbahar J, Rezaian G, Bararpour H. Distribution of plasma total homocysteine concentrations in the healthy Iranians. Clinical biochemistry, 2004, 37:149–51.
- Panagiotakos DB et al. The association between coffee consumption and plasma total homocysteine levels: the “ATTICA” study. Heart and vessels, 2004, 19(6):280–6.
- Strassburg A et al. Effect of age on plasma homocysteine concentrations in young and elderly subjects considering serum vitamin concentrations and different lifestyle factors. International journal vitamin and nutrition research, 2004, 74(2):129–36.
- Ueland PM, Monsen AL. Hyperhomocysteinemia and B-vitamin deficiencies in infants and children. Clinical chemistry and laboratory medicine, 2003, 41(11):1418–26.
- Bates CJ et al. Correlates of plasma homocysteine, cysteine and cysteinyl-glycine in respondents in the British National Diet and Nutrition survey of young people aged 4–18 years, and a comparison with the survey of people aged 65 years and over. British journal of nutrition, 2002, 87:71–9.
- Osganian SK et al. Distribution of and factors associated with serum homocysteine levels in children. Child and Adolescent Trail for Cardiovascular Health. Journal of the American Medical Association, 1999, 281:1189–96.
- Delvin E et al. Influence of methylenetetrahydrofolate reductase genotype, age, vitamin B-12, and folate status on plasma homocysteine in children. American journal of clinical nutrition, 2000, 72:1469–73.
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. British medical journal, 2002, 325:1202–9.
- Gerdes VE et al. Homocysteine and markers of coagulation and endothelial cell activation. Journal of thrombosis and haemostasis, 2004, 2:445–51.
- Mohanty D et al. Fibrinolysis, inhibitors of blood coagulation, and monocyte derived coagulant activity in acute malaria. American journal of hematology, 1997, 54(1):23–9.
- Hung CJ et al. Plasma homocysteine levels in Taiwanese vegetarians are higher than those of omnivores. Journal of nutrition, 2002, 132(2):152–8.
- Cunha AL et al. Metabolic effects of C677T and A1298C mutations at the MTHFR gene in Brazilian children with neural tube defects. Clinica chimica acta, 2002, 318:139–43.
- Rogers LM et al. High prevalence of cobalamin deficiency in Guatemalan schoolchildren: associations with low plasma holotranscobalamin II and elevated serum methylmalonic acid and plasma homocysteine concentrations. American journal of clinical nutrition, 2003, 77:433–40.


