M.H. Rahimi Rad1 and A. Hamzezadeh2
ABSTRACT To determine the prevalence and risk factors of wheeze, eczema and rhinitis in 6–7-yearold schoolchildren in Urmia, the International Study of Asthma and Allergies in Childhood questionnaire was given to the parents of 2999 student. Prevalence rates for wheeze, rhinitis and eczema in the previous 12 months were 9.4%, 9.8% and 2.7% respectively. The parents of only 12.8% of the children with current wheeze reported them as having asthma. The main risk factors for wheeze were male sex, presence of rhinitis or eczema, and smoking in the home. Prevalence of asthma and other allergic disease was lower than in industrialized countries and underdiagnosis of asthma was common.
Maladies allergiques chez les écoliers de 6 et 7 ans à Ourmia (République islamique d’Iran)
RÉSUMÉ Afin de déterminer la prévalence et les facteurs de risque de respiration sifflante, d’eczéma et de rhinite chez les écoliers d’Ourmia âgés de 6 et 7 ans, le questionnaire de l’étude internationale de l’asthme et des allergies chez l’enfant (International Study of Asthma and Allergies in Childhood) a été distribué aux parents de 2999 élèves. Les taux de prévalence de la respiration sifflante, de la rhinite et de l’eczéma au cours des 12 mois précédents étaient respectivement de 9,4 %, 9,8 % et 2,7 %. Les parents de 12,8 % seulement des enfants présentant une respiration sifflante ont déclaré que ceux-ci souffraient d’asthme. Les principaux facteurs de risque de la respiration sifflante étaient l’appartenance au sexe masculin, la présence de rhinite ou d’eczéma et le tabagisme au domicile. La prévalence de l’asthme et des autres maladies allergiques était plus faible que dans les pays industrialisés et l’asthme était fréquemment sous-diagnostiqué.
1Department of Respiratory Medicine; 2Department of Management and Human Resources, Urmia University of Medical Sciences, Urmia, Western Azerbaijan, Islamic Republic of Iran (Correspondence to M.H. Rahimi Rad:
Received: 22/02/06; accepted: 25/06/06.
EMHJ, 2008, 14(5):1044-1053
Introduction
Over the past few decades, the prevalence of allergic diseases has increased worldwide, especially in the more developed countries, affecting around 30% of the population [1,2]. The variety of methods used in epidemiological studies makes direct comparisons of results difficult. There are 2 standardized studies which were designed for the investigation of asthma and allergic disease: the European Community Respiratory Health Survey (ECRHS) [3,4] for adults and the International Study of Asthma and Allergies in Childhood (ISAAC) for children [5]. The ISAAC was designed to investigate childhood asthma, allergic rhinoconjunctivitis and eczema at the population level, with the aim of making international comparisons of the prevalence and severity of these disorders, thereby forming the basis for studies investigating the role of possible modifiable environmental factors that may ultimately lead to a reduction in the personal burden of these diseases [5]. In the first phase of the ISAAC, it was observed that wide variations exist between countries regarding the prevalence of these conditions, their clinical presentation and their natural history [6–8]. Among countries of the World Health Organization (WHO) Eastern Mediterranean Region participating in the ISAAC Phase I, the prevalence of a particular asthma symptom (current wheezing) ranged from 5.3% to 8.8% in children aged 6–7 years, and from 5.6% to 17.0% in children aged 13–14 years [7].
Since there is a paucity of epidemiological data on asthma and other allergic disease in the Islamic Republic of Iran, a country in Eastern Mediterranean Region, we conducted community-based studies in Urmia to determine the prevalence of allergic disease among children 6–7 years and 13–14 years and adults 20–44 years according to international protocols. In 2 previously published papers, current prevalence of wheezing in the previous 12 months was 14.5% among 13–14-year-old schoolchildren and 4.5% among 20–44-year-old adults [9,10] In this paper we report our analysis of the ISAAC Phase I written questionnaire survey of 6–7-year-old schoolchildren in Urmia. We also analysed additional data regarding factors which could possibly contribute to the prevalence of allergic disease in children.
Methods
This study was carried out in Urmia, the capital of West Azerbaijan province, in the north west of the Islamic Republic of Iran. Although it is not an industrial city, the majority of the cars are old with inefficient engines: the excessive tail-pipe emissions are regarded as an important source of air pollution [11].
Sample
In accordance with the ISAAC committee recommendation, we used a sample size of 3000 schoolchildren [5].
We divided Urmia city into 11 homogenous clusters and selected 4 clusters by random sampling. We selected a total of 25 schools by systematic random sampling in each selected cluster.
In the education year September 2002– May 2003, parents of participating children (all students aged 6–7 years in the first grade) were invited to the school and asked to complete questionnaires. A total of 3225 questionnaires were distributed. Trained medical students, research committee members, discussed the questionnaire with them and were in continuous contact with schools to address any questions the parents might have had. Extra sessions and interviews were organized for children of parents who were illiterate and not able to complete the questionnaires themselves.
Questionnaire
We used a Farsi translation of the ISAAC questionnaire for 6–7-year-old children. This had already been translated by the Iranian ISAAC coordination team and used in the Islamic Republic of Iran as part of the ISAAC [6–8]. The questionnaire is presented in 3 modules: the first includes 8 questions related to asthma; the second includes 6 questions related to rhinitis and the last includes 7 questions related to atopic eczema. The first 2 questions in each module discuss symptom prevalence, both at any time during the child’s life and in the preceding 12 months, which diminishes the possibility of recollection errors and provides greater specificity to the question in order to determine allergic disease prevalence. Question number 6, regarding asthma and rhinitis, and number 7, regarding eczema, aim to evaluate prior medical diagnoses. Some questions are used to evaluate symptom severity, such as those which evaluate the presence of speech limitations due to asthma symptoms, compromise of the child’s activities due to rhinitis symptoms, and sleep alterations owing to pruritus in cases of eczema.
Statistical analysis
The data from the questionnaires were double entered and analysed using SPSS for Windows, version 11. Prevalence for each of the symptoms was determined. We made a comparison between boys, girls and some risk factors using the chi-squared test. Pvalue < 0.05 was considered significant.
Results
We distributed 3225 questionnaires, and after 3 follow-ups and extra sessions with parents unable to complete the questionnaire, 3043 were returned (94% response rate); 44 of these were incomplete and therefore excluded from the analysis. The questionnaires for 2999 individuals (1450 boys and 1549 girls) were analysed.
Table 1 shows the prevalence of symptoms regarding asthma, allergic rhinoconjunctivitis and eczema. Prevalence of current wheezing was 9.4% and ever diagnosed asthma was 1.6%. Rhinoconjunctivitis was reported for 2.9% of children and ever diagnosed hay fever for 3.6%. There was history of chronic recurrent rash in 4.5% of the children; itchy rash affected identified parts of the body (such as the folds of the elbows and knees, in front of the ankles, under the buttocks or around the neck, ears or eyes) in 2.1%.
Table 2 shows the severity of asthma, allergic rhinoconjunctivitis and eczema in the present investigation. Asthma symptoms affected the daily activities of the children: prevalence of wheezing attacks > 12 times in the previous 12 months 0.7%, sleep disturbed by wheezing ≥ 1 time per week 2.0% and speech limited by wheezing in the previous 12 months 1.9%. In the past 12 months, rhinoconjunctivitis symptoms affected 5.7% of the students in their daily activities and eczema rash woke 2.1% of the children during their sleep.
To facilitate comparison we included published global and Regional means for asthma variables [6] in Table 3. Generally, prevalence for asthma variables in Urmia was lower than the global mean except for attacks and wheeze disturbs sleep, but higher than the WHO Eastern Mediterranean Region mean except for night cough and asthma ever. In general, more children had respiratory and allergic rhinitis symptoms than had medically diagnosed illness. Of 477 students reported as having wheezing ever, only 45 (9.4%) had history of diagnosed asthma. Of 281 students with history of wheeze in the previous year 36 (12.8%) had diagnosis of asthma.
Wheezing ever (15.9%) was the most common symptom. Boys were significantly more likely than girls to have asthma, wheeze ever, current wheeze, exercise induced wheeze, night time cough, rhinitis ever, current rhinitis, and hay fever. There were no significant sex differences for eczema or chronic rash.
Figure 1 shows the overlap of asthma, eczema and allergic rhinitis. While parents of 12.9% of the 6–7-year-olds in this study reported that their child had ≥ 1 of these conditions in the past 12 months, only 0.13% reported having all 3.
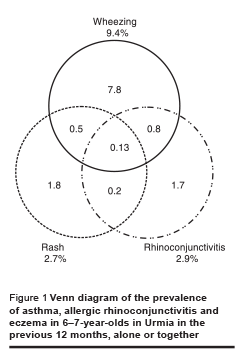
Table 4 shows the risk factors with positive association with wheezing ever and current wheezing. While maternal smoking was significantly associated with wheezing ever and current paternal smoking had weakly positive association. There was no association between wheeze, current or ever, and mother’s education level, house heating system (electricity, gas or wood), keeping a dog, sibship size, living near busy roads or acetaminophen ingestion.
Discussion
The use of the standard ISAAC protocol permits comparison of our results in Urmia with other cities and countries.
The summary results of phase 1 of the ISAAC survey documents asthma symptoms in 91 centres in 38 countries in 6–7year-old schoolchildren [6]. For wheezing in the previous 12 months, there was a > 5fold difference in prevalence between countries (4.1%–32.1%), with lowest rates in India (5.6%), Indonesia (4.1%) and Islamic Republic of Iran (5.4%) and highest rates in Australia (24.6%), Costa Rica (32.1%) and New Zealand (24.5%). The range in prevalence of “asthma ever” was very large, ranging from 1.4%–2.5% in Estonia, Latvia and Poland to 26.5%–27.1% in Australia, Costa Rica and New Zealand.
In studies carried out as part of the ISAAC protocol in Tehran, prevalence of current wheezing was 5.5% in 1994 [8] and 8.6% in 2002 [12], lower than the 9.4% in our study.
Prevalence of asthma in developing countries, characteristically low for a long time, has recently increased following the urbanization and the industrialization process. Possible explanations have been identified in the sudden exposure to pollution from industrial and motor vehicle exhaust emissions as a consequence of urbanization; changes in diet that caused a loss of protection against allergic diseases; and decrease in Ascaris lumbricoides infections, considered by some to bestow protection against the development of asthma [13]. The effects of all these factors, and of many others, may be more important in younger subjects.
Underdiagnosis of asthma
The number of parents who were aware that their child had asthma diagnosis lagged well behind the cumulative prevalence of wheezing. Of children with wheeze in the previous year, 87.8% had no diagnosis of asthma. Although this might be partly explained by causes of wheezing other than asthma, the most likely explanation for most of this deficit is underdiagnosis of the condition or failure to give a diagnosis of asthma.
This deficiency stems from not recognizing atypical or less common presentations of the condition such as cough and symptom suggestive of bronchial hyperactivity or from a reluctance to label a child as suffering from asthma. In a study in North Carolina using the ISAAC protocol, 17% reported current asthma-like symptoms without diagnosis of asthma [1]. In comparison with that study, underdiagnosis in our study was very high (87.8%). In an ISAAC steering committee study, the correlation between the proportion of the population wheezing in the previous 12 months and the proportion reporting asthma ever was 0.73 [6]. In some countries the reporting of 12-month wheeze was much higher than the reporting of “asthma ever” (e.g. Brazil 23.3% versus 13.1%; Estonia 9.3% versus 1.4%; Islamic Republic of Iran 5.4% versus 3.0%), whereas in other countries the opposite was true (e.g. Philippines 11.3% versus 16.4%; Oman 7.1% versus 10.5%).
Higher prevalence in males
In general, in our study, boys had a higher prevalence of medical diagnosis and symptoms than girls, with the exception of eczema and eczema-related symptoms. This is similar to an ISAAC steering committee study [5] in which males showed a significantly higher prevalence than females in this age group (P < 0.001) for 12 month wheezing (1:0.81), ≥ 4 attacks of wheezing per week (1:0.76), waking on ≥ 1 nights a week (1:0.92), wheezing severe enough to limit speech (1:0.78), wheeze with exercise (1:0.79) and dry cough at night (1:0.92). There were no significant differences between countries.
After puberty, asthma becomes more prevalent in females: by adulthood, the sex ratio (female:male) of incidence of asthma admissions is 3:1 in the United States of America [13].
Risk factors and asthma
There was significant association with cat ownership and wheezing. The association between exposure to pets early in life and allergic disease remains controversial. Indoor exposure to mite (Dermatophagoides farinae) antigen 1 and cat antigen d1 is a risk factor for respiratory symptoms in adults, and for cat antigen d1 even in nonsensitized individuals. The risk is increased if subjects are exposed to a mixture of allergens or if they are sensitized in addition to high exposure [14]. Pet exposure during the first year of life has been associated with a lower prevalence of asthma in large population-based samples [15,16]. It has been hypothesised that childhood exposure to pets, including cats, might modulate immunological mechanisms and reduce sensitization to cats in adult. In this study no association was found between keeping a dog and wheezing: the small proportion of participants keeping dogs was probably the reason for this. A negative association between dog ownership and the development of atopic diseases in early childhood has been observed but only in families without a history of atopic disease [14,17].
There was a positive association between maternal smoking and prevalence of wheezing ever and current wheeze. Paternal smoking was weakly associated with wheezing ever but not with current wheezing. In children, there is convincing evidence that parental, especially maternal, smoking increases susceptibility to lower respiratory illness in infants and causes chronic respiratory symptoms [18]. A largescale ISAAC study on 23 044 children aged 6 to 15 years suggested that environmental tobacco smoke might be associated with an increased prevalence of wheeze and asthma in Japanese children [19].
Rhinitis and eczema ever were strongly associated with wheezing currently and ever. It has been reported that atopic dermatitis and rhinitis are more common in people with asthma. The risk for asthma attributable to atopy has been estimated at 30% [13].
There was no significant association between residence near a busy road and current and ever wheezing, but a previous study in 13–14-year-olds in Urmia showed an association [9]. This finding is compatible with Wieringa et al., who observed a higher occurrence of asthma symptoms in an urban than a suburban area in adults but not in children, perhaps as a result of the progressive effect of long-term exposure to the urban environment [20].
In this study there was positive association between antibiotic use in the previous year and wheezing. It has been hypothesized that antibiotic use early in life may increase the subsequent risk of asthma. An ecologic analysis of the relationship between antibiotics sales and the prevalence of asthma symptoms, allergic rhinoconjunctivitis, and atopic eczema in 99 centres from 28 countries was generally not consistent with the hypothesis [21].
In our study there was no association between sibship size and wheezing. Recent epidemiological studies consistently report an inverse association between sibship size and allergic disease [22].
The present results indicate that exposure to cockroaches was significantly associated with asthma among the children studied and can be considered a risk factor for the disease. It has been found that there are more asthma sufferers in homes with high cockroach infestation [23]. Based on data reported in a study in the United States of America, Arruda et al. reported that the combination of exposure and sensitivity to cockroach allergens is a risk factor for the severity of asthma in children [24].
There are some reports that frequent acetaminophen (paracetamol) use was positively associated with asthma and rhinitis in 13–14-year-old students and adults [25,26]. Results of a previous study in Urmia on 13–14-year-old children are consistent with those reports but in this study we did not find a positive association with wheezing [9].
Prevalence of rhinitis
Again in this age group boys suffered more than girls with symptoms of rhinitis. An ISAAC study in 257 800 children aged 6–7 years from 91 centres in 38 countries showed that the prevalence of rhinitis with itchy/watery eyes (rhinoconjunctivitis) in the previous year varied between centres from 0.8% to 14.9%. The lowest prevalence was found in parts of Eastern Europe and south and central Asia [7]. Mirsaid Ghazi et al. reported prevalence of allergic rhinitis in Tehran at 23.28% in 10–14-year-old students, and this was more common in boys [27].
An itchy rash which had been coming and going for at ≥ 6 months, which was meant to be suggestive of eczema, was the least commonly occurring of the 3 allergic conditions investigated in this study. Surprisingly, prevalence of diagnosed eczema was greater than prevalence of eczema symptoms. For explanation one could speculate that in Iran most skin lesions are labeled as eczema, however the most probable explanation is that since there is no equivalent Farsi wording for “eczema”, we used “skin sensitivity” in parenthesis in the translated form of the question “Have you ever had eczema?” This led to a high response rate to the question. In a similar study in Bangkok, university students’ use of “allergic rash” for eczema led to an overestimation [28]. The ISAAC steering committee study [8] showed a prevalence range for symptoms of atopic eczema from < 2% in the Islamic Republic of Iran to > 16% in Japan and Sweden in the 6–7-year-old age group.
The ISAAC study includes 6–7and 13–14-year-old schoolchildren. There are, however, fewer reports in the literature for the younger age group. Data from our study will serve as a baseline for prevalence of major allergic disease among 6–7-year-old schoolchildren in the Islamic Republic of Iran.
A major limitation of this study was that apart from the information given on the questionnaire no measurements of atopic disease, such as skin-prick test, measurement of IgE concentration or lung function, were performed. ISAAC questionnaires were, however, specially designed for population-based surveys and have been shown to be valid instruments.
Acknowledgements
We would like to thank the schools, parents and children who participated in the research, the medical students who helped to collect the data, Urmia University of Medical Sciences Research Committee, which approved the study, and the Iranian ISAAC team who provided translated ISAAC questionnaires.
References
- Magnus P, Jaakkola JJ. Secular trend in the occurrence of asthma among children and young adults: critical appraisal of repeated cross sectional surveys. British medical journal, 1997, 314:1795–9.
- Aberg N et al. Increase of asthma, allergic rhinitis and eczema in Swedish schoolchildren between 1979 and 1991. Clinical and experimental lllergy, 1995, 25:815–9.
- Burney PG et al. The European Community Respiratory Health Survey. European respiratory journal, 1994, 7:954–60.
- No authors listed. Variations in the prevalence of respiratory symptoms, selfreported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS). European respiratory journal, 1996, 9(4):687–95.
- Asher M et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. European respiratory journal, 1995, 8(3):483–91.
- Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC). European respiratory journal, 1998, 12(2):315–35.
- Strachan D et al. Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC). Pediatric allergy and immunology, 1997, 8(4):161–76.
- Williams H et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. Journal of allergy and clinical immunology, 1999, 103(1 Pt 1):125–38.
- Rahimi Rad MH, Hejazi ME, Behrouzian R. Asthma and other allergic diseases in 13–14-year-old schoolchildren in Urmia: an ISAACC study. Eastern Mediterranean health journal, 2007, 13(5):1005–16.
- Rahimi-Rad MH, Gaderi-Pakdel F, Salari-Lak S. Smoking and asthma in 20–44-year-old adults in Urmia, northwest Islamic Republic of Iran. Eastern Mediterranean health journal, 2008, 14(1):6–16.
- US Department of Energy. Iran pollution report. Tehran, Alexander Aghayan & Associates, Inc., 2002 (http://www.aghayan. com/iranpol0502.htm, accessed 23 January 2008).
- Masjedi M et al. Prevalence and severity asthma symptoms in children of Tehran. Iranian journal of allergy, asthma & immunology, 2004, 3:25–30.
- Viegi G, Annesi-Maesano I, Matteeli G. Epidemiology of asthma. In: Chung F, Fabbri LM, eds. Asthma. European respiratory monograph, 2003, 23:1–25.
- Gehring U et al. Respiratory symptoms in relation to indoor exposure to mite and cat allergens and endotoxins. European respiratory journal, 2001, 18:555–63.
- Roost HP et al. Role of current and childhood exposure to cat and atopic sensitization. European Community Respiratory Health Survey. Journal of allergy and clinical immunology, 1999, 104(5):941–7.
- Hesselmar B et al. Does early exposure to cat or dog protect against later allergy development? Clinical and experimental allergy, 1999, 29(5):611–7.
- Pohlabeln H, Jacobs S, Böhmann J. Exposure to pets and the risk of allergic symptoms during the first 2 years of life. Journal of investigative allergology & clinical immunology, 2007, 17(5):302–8.
- Stocks J, Dezateux C. The effect of parental smoking on lung function and development during infancy. Respirology, 2003, 8:266–85.
- Tanaka K et al. Prevalence of asthma and wheeze in relation to passive smoking in Japanese children. Annals of epidemiology, 2007, 17(12):1004–10.
- Wieringa MH et al. Higher occurrence of asthma-related symptoms in an urban than a suburban area in adults, but not in children. European respiratory journal, 2001, 17(3):422–7.
- Foliaki S et al. Antibiotic sales and the prevalence of symptoms of asthma, rhinitis, and eczema: The International Study of Asthma and Allergies in Childhood (ISAAC). International journal of epidemiology, 2004, 33(3):558–63.
- Kinra S et al. Association between sibship size and allergic diseases in the Glasgow Alumni Study. Thorax, 61(1):48–53.
- Sarinho E et al. There are more asthmatics in homes with high cockroach infestation. Brazilian journal of medical and biological research, 2004, 37(4):503–10.
- Arruda LK et al. Cockroach allergens and asthma. Journal of allergy and clinical immunology, 2001, 107(3):419–28.
- Davey G et al. Use of acetaminophen and the risk of self-reported allergic symptoms and skin sensitization in Butajira, Ethiopia. Journal of allergy and clinical immunology, 2005, 116(4):863–8.
- Newson RB et al. Paracetamol sales and atopic disease in children and adults: an ecological analysis. European respiratory journal, 2000, 16(5):817–23.
- Mirsaid Ghazi B et al. Frequency of allergic rhinitis in school-aged children (7–18 years) in Tehran. Iranian journal of allergy, asthma & immunology, 2003, 2:181–4.
- Vichyanond P et al. Prevalence of asthma, allergic rhinitis and eczema among university students in Bangkok. Respiratory medicine, 2002, 96(1):34–8.


