M.K. Daboubi1 and B. Khreisat1
1Department of Gynaecology and Obstetrics, King Hussein Medical Centre, Amman, Jordan (Correspondence to M.K. Daboubi:
Received: 04/12/05; accepted: 09/03/06
EMHJ, 2008, 14(4):972-977
Introduction
Massive ovarian oedema is an uncommon cause of adnexal mass, first described by Kalstone in 1969 [1–7]. Massive solid enlargement of the ovary can be associated with interstitial oedema without neoplastic changes and is considered to be the result of torsion of the ovary. This can be easily mistaken for neoplasm, in which case the appropriate treatment will be removal of the whole ovary. However, when this condition is a benign enlargement of the ovary it needs conservative intervention. The group most commonly affected are young women in the child-bearing period, but a few cases have been described in prepubertal girls. Masculinization is a common feature of many adult cases, but there has been a case with precocious puberty as the presenting symptom [8,9], and it has been described during pregnancy [10]. Geist et al. stated that massive oedema of the ovary is a rare entity affecting mainly young women [2]. It is important to recognize the condition as it is often misdiagnosed for a malignancy, putting the younger patient at risk of over-treatment with the resultant loss of hormo-nal function and fertility.
We present the case of a 30-year-old woman with findings of massive ovarian oedema on ultrasound imaging, and a discussion and literature review aiming to clarify that this is a benign tumour-like condition which every gynaecologist can encounter and that the most appropriate treatment is conservative surgery. A Med-line search on PubMed since 1969 using the keywords [massive ovarian oedema case report] found around 117 cases reported.
Case report
Our case was a 30-year-old Jordanian wo-men married as a second wife to a Jordanian man for the previous 8 months, gravida 1, para 0, with reported history of no contraception use. Her menstrual cycles were regular with menarche at the age of 12 years. She had an uneventful past medical history.
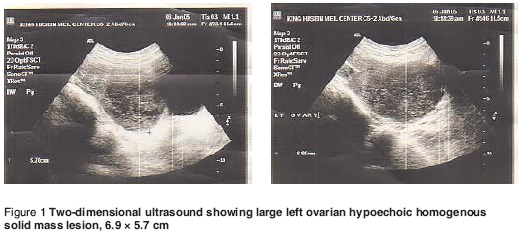
This woman was referred to our clinic with chronic lower abdominal pain for the previous 3 months. The pain was mild, colicky, mainly on the left lower abdomen which occasionally referred to the back with no other associated symptoms. Physical examination showed findings of mild lower abdominal tenderness on the left side with a tender mobile adnexal mass felt during bimanual examination. Ultrasound examination showed a large left ovarian hypoechoic homogenous solid mass lesion 7 × 6 cm (Figure 1), which showed poor vascularity on colour Doppler scan. There was no ascitic fluid seen. The serum level of CA125, the marker for ovarian cancer, was 27.56 U/mL, beta-human chorionic gonadotropin (β-HCG) < 5 μ/mL, luteinizing hormone (LH) 15.4 mIU/mL, follicle stimulating horomine (FSH) 11.4 mIU/ml and, α-fetoprotein 1.14 ng/mL; progesterone, oestradiol and testosterone levels were within normal values.
Laparotomy was performed with fin-dings of a grossly enlarged left ovary (8 × 6 cm), twisted once, intact smooth shiny white capsule, oedematous left tube, with normal right tube and ovary. Other pelviabdominal organs were normal. When the capsule of the left ovary was incised, copious amounts of clear fluid emerged and bulging was observed at the cut surface. Our previous readings on these findings raised the possibility of ovarian oedema as the condition. More than a quarter of the ovary was removed by wedge resection and was sent for frozen sectioning. Ovarian oedema was confirmed. Later histopathological examination showed normal germinal follicles, multiple follicular cysts with normally developed theca interna and externa, and some follicles showing an attenuated layer of cells. The ovarian stroma showed extensive oedema. The ovary was sutured and fixed to the postlateral aspect of the uterus near to the left ovary.
The patient showed marked improve-ment in her pain as the main symptom, and was discharged on oral contraceptive pills. One week later the patient was seen at the clinic, where transvaginal ultrasound showed a marked decrease in the size of the left ovary back to normal (3.5 × 2.2 cm). One month later, after stopping the course of oral contraceptives, the patient became spontaneously pregnant, which continued to term and delivery of a healthy female baby weighing 3.35 kg.
What is special in our case is our previous knowledge of the condition, the ultrasound findings and the clinical features which made the diagnosis and thus the management straightforward with wedge resection, frozen section and fixation of the ovary to the uterus followed by successful pregnancy.
Discussion
Massive solid enlargement of the ovary is a tumour-like condition occurring in young women [1], considered to be the result of torsion of the ovary to the extent that it interferes with venous and lymphatic drainage but is insufficient to cause necrosis [4,5]. However, few case studies have described any histopathological evidence of haematoma, which is usual with even partial torsion [5]. Most authors suggest that partial torsion is a likely explanation for this perplexing disorder [5] and it may be a variant of polycystic ovary syndrome [6]. Marked enlargement of the ovary occurs and the patient usually presents with adnexal mass. If torsion occurs acute abdominal pain is prominent. Menstrual irregularities, infertility and abdominal distension are found in the majority of cases [5].
Masculanization is a common feature of many adult cases [4–7], precocious puberty was the presenting symptom in some prepubertal girls [8,9], and other cases presented with vaginal bleeding or masculanization associated with low serum levels of gonadotropins, indicating autonomous ovarian hormone production [10,11]. This hormone production is due to stromal luteinization as suggested by Chervenak [10]. Kalstone suggested that the luteinization might be caused by the mechanical stimulus of stretching the stroma by oedema fluid [1]. Another explanation for the oedema and abnormal hormone production is a derangement of a local paracrine factor, such as insulin-like growth factor, epidermal growth factor or cytokines [5]. Massive ovarian oedema due to permeation of the ovarian lymphatics by metastatic carcinoma is rare, with a few cases reported to date [3].
From a Medline review of the world literature, it seems that the group most commonly affected is young postmenarchal women. Some cases have been described in prepubertal girls [4,10] and 1 menopausal woman [12], another in a girl aged 6 months [8].
The ultrasounic findings have been reported to be a solid tumour-like hypo-echoic homogenic mass or as a solid mass containing a cystic component. Umesaki et al. suggest that the ultrasound detection of multiple peripheral ovarian follicles in a solid ovarian tumour-like mass may make the preoperative diagnosis of massive ovarian oedema possible by ultrasound alone [13]. Recent reports using magnetic resonance imaging (MRI) have demonstrated that multiple ovarian follicles situated around the periphery of the cortex of the enlarged ovary are the most important indicator of massive ovarian oedema [13]. A potential role of MRI in preoperative diagnosis of the condition is suggested although diagnosis is possible by ultrasound alone [13–16].
Morphological recognition of the lesion is fairly simple. The cut surface of the specimen appears grey in colour, wet and soft and the oedema fluid oozes out with a bulge after cutting with a knife due to the pressure of the oedema.
Histopathology features
The ovarian stromal cells are widely separated by copious oedema fluid and atretic follicles may at times be recognized. The tunica albuginea and superficial cortical zone are characteristically uninvolved [4]. A thin rim of compressed cortical stroma is recognized at the periphery of the mass. Necrosis and haemorrhage are unusual.
Focal stromal leutinization has been noted in some of the studied cases, and is thought to be a mechanical process induced by stretching of the stromal cells [9].
In a case studied by electron microsco-py, Ratel et al. reported that the principal finding was the presence of both fibroblasts and myofibroblasts in the oedematous stroma [4]. The increased number of myofibroblasts may be a response to the oedema.
The great majority of cases are unilateral and the most common treatment is unilateral salpingo-oopherectomy [16], as the lesions are mistaken for primary ovarian neoplasms at laparotomy. Conservative treatment must be the rule [17], especially since the disorder is benign. After extensive review of the literature, Geist et al. concluded that most cases were over-treated, and this entity should be suspected in women in the fertile age range with solid enlargement of the ovary, and definite treatment should be undertaken only after confirmed patholo-gical diagnosis. Conservative treatment is feasible and should be the rule in these cases, where preservation of fertility is mandatory [2].
However, when the condition of ovarian oedema is suspected at surgery the appropriate treatment is wedge resection, removing 30% or more of the ovary to exclude secondary causes of the condition. Frozen section is valuable at the time of surgery. We emphasize wedge resection with fixation of the ovary to the uterus to prevent further torsion. Laparoscopic conservation of the ovaries in cases with massive ovarian oedema has been reported [18]. With the laparoscope it is possible to diagnose the enlarged ovary, grey in colour with twisted pedicle and intact capsule, and to untwist the ovary, fix it to the posterior wall of the uterus and to take a biopsy from the ovary.
In our case, when the condition of ovarian oedema was suspected prior to surgery due to our previous experience with a case [16], the treatment at surgery was wedge resection of the ovary and frozen sectioning to exclude secondary causes of ovarian oedema [19]. Fixation of the ovary to the uterus was done to prevent torsion, as some authors suggested [5,16].
In summary, massive ovarian oedema can occur as a primary or secondary oedema:
Primary oedema occurs when the ovary is not diseased and when there is torsion or twisting of the ovarian pedicle to the extent that it interferes with the venous blood supply leading to oedema and does not affect the arterial blood flow. Incomplete or intermittent torsion can occur.
Secondary massive ovarian oedema can occur secondary to a diseased ovary, such as when there is:
Ovarian mass and cyst.
Malignancy. There are reports of lymphatic permeation by metastatic carcinoma from the uterine cervix [3], with mature cystic teratoma [20], gastric carcinoma [21], ovarian fibrothecoma [22], lymphangitis car-cinomatosa [23] and Meig syndrome [24].
Fibromatosis. Fibromatosis and mas-sive oedema of the ovary are possibly related entities, as discussed in a report of 14 cases of fibromatosis and 11 cases of massive oedema [25]. The similar age range and clinical manifestations of these 2 processes and the overlap in their histological features suggest that they are closely related and may reflect differing morphologic expressions of the same underlying disorder. Some of the cases of massive oedema, however, may result from the development of stromal oedema in ovaries involved by hyperthecosis.
Polycystic ovary [26].
Drugs for induction of ovulation [27].
In summary, massive ovarian oedema is a rare cause of ovarian mass. The appropriate therapy in most cases is wedge resection with fixation of the ovary to the uterus. Ovarian oedema could occur as a primary condition with twisted ovarian pedicle or secondary to a diseased ovary.
References
- Kalstone CE, Jaffe RB, Abell MR. Mas-sive edema of the ovary simulating fibroma. Obstetrics and gynecology, 1969, 34:564–71.
- Geist RR et al. Massive edema of the ovary: a case report and review of the pertinent literature. Journal of pediatric and adolescent gynecology, 2005, 18(4):281–4.
- Krasevic M et al. Massive edema of the ovary. A report of two cases due to lymphatic permeation by metastatic carcinoma from the uterine cervix. Gynecologic oncology, 2004, 93(2):564–7.
- Roth LM, Deaton LM, Sternberg WH. Mas-sive ovarian edema. A clinicopathological study of five cases including ultra struc-tural observations and review of the literature. American journal of surgical pathology, 1979, 3(1):11–21.
- Eden JA. Massive ovarian oedema. British journal of obstetrics and gynaecology, 1994, 101:456–8.
- Roth LM. Massive ovarian edema with stromal luteinization: a newly recognized virilizing syndrome apparently related to partial torsion of the mesovarium. American journal of clinical pathology, 1971, 55:757–60.
- Siller BS et al. Massive edema of the ovary associated with androgenic mani-festations. Southern medical journal, 1995, 88(11):1153–5.
- Natarajan A et al. Precocious puberty secondary to massive ovarian edema in a 6-month-old girl. European journal of endocrinology, 2004, 150(2):119–23.
- Kanumakala S et al. Massive ovarian edema causing early puberty. Journal of pediatric endocrinology and metabolism, 2002, 15(6):861–4.
- Chervenak FA et al. Massive ovarian edema: review of world literature and report of two cases. Obstetrical & gynecological survey, 1980, 35:677–84.
- Vasquez SB, Sotos JF, Kim MH. Massive edema of the ovary and virilization. Obstetrics and gynecology, 1982, 59(6 Suppl.):95S–99S.
- Shirk JO, Copas PR, Kattine AA. Massive ovarian edema in a menopausal woman. A case report. Journal of reproductive medicine, 1996, 41(5):359–62.
- Umesaki N et al. Sonographic charac-teristics of massive ovarian edema. Ultrasound in obstetrics & gyneco-logy, 2000, 16(5):479–81.
- Kramer LA, Lalani T, Kawashima A. Mas-sive edema of the ovary: high resolution MR findings using a phased-array coil. Journal of magnetic resonance imaging, 1997, 7(4):758–60.
- Roberts CL, Weston MJ. Bilateral mas-sive ovarian edema: a case report. Ultra-sound in obstetrics & gynecology, 1998, 11(1):65–7.
- Daboubi MK et al. Massive ovarian oede-ma: a case report. Jordan medical journal, 1995, 29(2):96–8.
- Mutijima E et al. Edeme massif de l’ovaire, a propos d'un cas [Massive ovarian edema]. Revue médicale de Liège, 2004, 59(9):522–4.
- Kocak M, Caliskan E, Haberal A. Laparos-copic conservation of the ovaries in cases with massive ovarian edema. Gynecologic and obstetric investigation, 2002, 53(2):129–32.
- Kleiner GK et al. Wedge resection in massive edema of the ovary. American journal of obstetrics and gynecology, 1978, 132:107–8.
- Lakhey M et al. Massive ovarian edema with contralateral mature cystic teratoma––a case report of an uncommon combination. Indian journal of pathology & microbiology, 2003, 46(2):219–21.
- Bazot M et al. Massive ovarian edema re-vealing gastric carcinoma: a case report. Gynecologic oncology, 2003, 91(3):648–50.
- Sakaki M et al. Ovarian fibrothecoma with massive edema. Journal of medical investigation, 2000, 47(3–4):148–51.
- Wong SY. Bilateral massive ovarian oedema—report of a case due to lymphangiitis carcinomatosa. Virchows Archiv A, Pathological anatomy and histopathology, 1989; 414(4):355–8.
- Lacson AG et al. Secondary massive ovarian edema with Meig’s syndrome. American journal of clinical pathology, 1989, 91(5):597–603.
- Young RH, Scully RE. Fibromatosis and massive edema of the ovary, possibly related entities: a report of 14 cases of fibromatosis and 11 cases of massive edema. International journal of gyneco-logical pathology, 1984, 3(2):153–78.
- Guvenal T, Cetin A, Tasyurt A. Unilateral massive ovarian edema in a woman with polycystic ovaries. European journal of obstetrics, gynecology, and reproductive biology, 2001, 99(1):129–30.
- Patty JR, Galle PC, McRae MA. Massive ovarian edema in a woman receiving clomiphene citrate. A case report. Journal of reproductive medicine, 1993, 38(6):475–9.


