K. Jabeen,1 A. Zafar1 and R. Hasan1
ازدياد استفراد النمط المصلي إينابا O1 من ضمات الكوليرا بدلاً من النمط المصلي أوغاوا في باكستان
رومينه حسن، كوثر جبين، عفيه ظَفَر
الخلاصـة: على الرغم من أن النمط المصلي السائد في باكستان من ضمات الكوليرا هو أوغاوا، وأن النمط المصلي إينابا نادر، فقد لوحظ ازدياد يعتد به إحصائياً في استفراد النمط المصلي إينابا في المختبر المرجعي الذي تعمل به الباحثات في كراتشي. وتقدم هذه الورقة تقريراً عن هذه الملاحظة مع تحليل إضافي للمعطيات السابقة والتي تعود إلى الفتـرة ما بين 1993 – 2005 حول الكوليرا، وذلك لتقييم اتجاه الحدوث ونمط مقاومة ذراري ضمات الكوليرا. وقد نمت 245 مزرعة من بين 3292 مزرعة (7.4%) لتنتج ضمات الكوليرا في الفتـرة بين كانون الثاني/يناير وأيلول/سبتمبر 2005 وكان 243 من بين تلك المزارع من النمط المصلي إينابا، وهو أضعاف ما نما من النمط المصلي أوغاوا. ولوحظ أن النمط المصلي إينابا الحالي مقاوم بنسبة 100% للكوتريموكسازول، وبنسبة 3% للكلورامفينيكول، وغير مقاوم للأمبيسيلين أو التتـراسيكلين أو الأوفلوكساسين، وأن نمط الاستجابة للمضادات الحيوية مماثل للنمط المصلي السابق والسائد أوغاوا.
ABSTRACT: Although the predominant Vibrio cholerae serotype in Pakistan is Ogawa and serotype Inaba is rare, there has been a significant increase in the isolation of Inaba in our referral laboratory in Karachi. This paper reports this observation and further analysis of previous cholera data from 1993 to 2005 to assess the trend of occurrence and resistance pattern of V. cholerae strains. From January to September 2005, 245/3292 (7.4%) specimens yielded growth of V. cholerae. Of these, 243 were serotype Inaba, outnumbering serotype Ogawa. This recent Inaba strain is 100% resistant to co- trimoxazole, 3% resistant to chloramphenicol and not resistant to ampicillin, tetracycline and ofloxacin. This sensitivity pattern is almost similar to that of the previous predominant serotype Ogawa.
Accroissement de l’isolement du sérotype Vibrio cholerae O1 Inaba par rapport au sérotype Ogawa au Pakistan
RÉSUMÉ: Bien que le sérotype de Vibrio cholerae prédominant au Pakistan soit Ogawa et que le sérotype Inaba soit rare, on a constaté une augmentation significative de l’isolement d’Inaba dans notre laboratoire de référence de Karachi. Le présent article porte sur cette observation ainsi que sur une analyse plus poussée des précédentes données sur le choléra pour la période 1993-2005, afin d’évaluer la tendance de l’apparition et le schéma de résistance des souches de V. cholerae. De janvier à septembre 2005, 245/3292 (7,4 %) échantillons ont donné une culture de V. cholerae. Sur ces échantillons, 243 étaient du sérotype Inaba, donc plus nombreux que le sérotype Ogawa. Cette récente souche d’Inaba est résistante à 100 % au cotrimoxazole, à 3 % au chloramphénicol et non résistante à l’ampicilline, à la tétracycline et à l’ofloxacine. Ce profil de sensibilité est pratiquement identique à celui du sérotype Ogawa autrefois prédominant.
1Department of Pathology and Microbiology, Aga Khan University, Karachi, Pakistan (Correspondence to K. Jabeen:
Received: 04/01/06; accepted: 09/03/06
EMHJ, 2008, 14(3):564-570
Introduction
Vibrio cholerae , the causative agent of cholera, is subdivided into serogroups based on the somatic O antigen. Only the O1 and O139 serogroups are reported to cause epidemic and pandemic disease. Each of the O1 biotypes can be further subdivided into 2 major serotypes, Ogawa (VCO) and Inaba (VCI). Hikojima, a 3rd serotype also exists but is rare and unstable [1]. V. cholerae O1 strains can undergo serotype conversion or switching between the Inaba and Ogawa serotypes related to a mutation in the wbeT region, a gene responsible for O1 antigen biosynthesis [2].
The predominant serotype in Pakistan is Ogawa and serotype Inaba is rare. Various centres from Pakistan have published reports about V. cholerae isolates and in all of them isolation of Inaba serotypes are infrequent [3–5]. In India and Bangladesh also the predominant serotype is Ogawa. However, 2 reports of the emergence of VCI have been published from India (Delhi and Chandigarh) showing increased isolation of VCI in their cholera isolates [6,7]. This phenomenon is not yet reported from Pakistan.
Recently, however, there has been a significant increase in the isolation of serotype Inaba in our laboratory, which has even outnumbered serotype Ogawa. This paper reports this observation and further analysis and comparison with previous cholera data at our institution to assess the trends of occurrence and resistance pattern of V. cholerae Ogawa and Inaba strains.
Methods
Setting
This descriptive study was conducted during 1993–2005 at Aga Khan University, a tertiary care centre located in Karachi, Pakistan. The microbiology laboratory of the university receives specimens from both inpatients and outpatients from clinics and hospitals within the city and all over the country.
Specimen selection
All stool samples yielding growth of V. cholerae were selected from both inpatients and outpatients. Data was retrieved from a centralized computer database. Duplicate specimens from the same patients were excluded.
Microbiological methods
All stool samples for the isolation of V. cholerae were plated on tellurite taurocholate gelatin agar (TTGA) and incubated at 37 ºC. In addition samples were inoculated in alkaline peptone water (APW) and after 6 hours of incubation at 37 ºC were further subcultured on TTGA. After 24 hours of incubation, suspect colonies from TTGA were confirmed as V. cholerae using standard methods [8]. Serogroups were identified by slide agglutination with polyvalent anti-sera for Ogawa and Inaba strains (Murex Diagnostic Limited), and for serogroup O139 (Dienka Sieken Co. Limited, Japan).
Antimicrobial susceptibility testing was performed by Kirby Bauer disc sensitivity technique on Mueller–Hinton agar [9]. Antibiotics that were tested included ampicillin (10 μg), tetracycline (30 μg), cotrimoxazole (1.25/23.75 μg), chloramphenicol (30 μg) and ofloxacin (5 μg). The zone sizes (mm) for resistant (R), indeterminate (I) and sensitive (S) strains were defined as follows: for ampicillin (R if ≤ 13, I if 14–16, S if ≥ 17), for tetracycline (R if ≤ 14, I if 15–18, S if ≥ 19), for co-trimoxazole (R if ≤ 10, I if 11–15, S if ≥ 16), for chloramphenicol (R if ≤ 12, I if 13–17, S if ≥ 18) and for ofloxacin (R if ≤ 12, I if 13–15 S if ≥ 16). Escherichia coli ATCC25922 was used as the control.
Statistical analysis
Data was entered and analysed using SPSS. P values were calculated using the t-test for 2 independent samples.
Results
From January to September 2005 a total of 3290 stool samples were received for culture at Aga Khan University laboratory. Of these 245 (7.4 %) yielded growth of V. cholerae. The total number of V. cholerae serotype Inaba (VCI) strains isolated were 243 which greatly outnumbered serotype Ogawa (VCO) (2 strains).
Over the period 1993–2005 the frequency of isolation of VCI varied from year to year (Figure 1). However, there was a significant upsurge in the years 2004–05. In 2004, isolation of VCI was first observed in the month of November and there was an increase in the trend of isolation of VCI over the months.
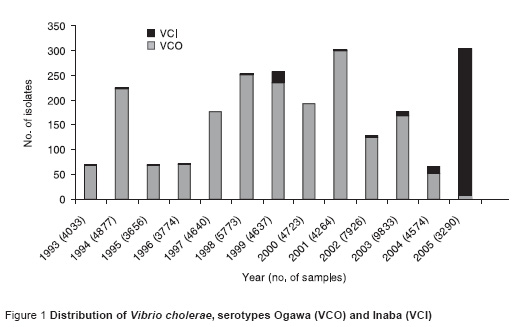
The mean age of patients with VCO was 20 years compared with 23 years for patients with VCI (P = 0.06).
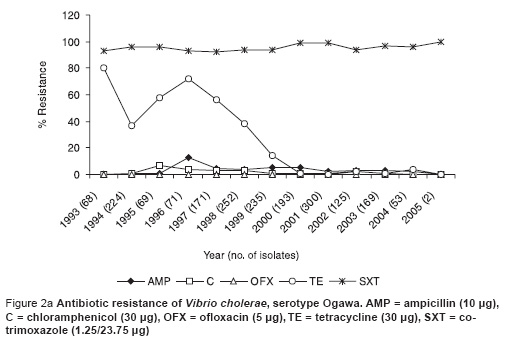
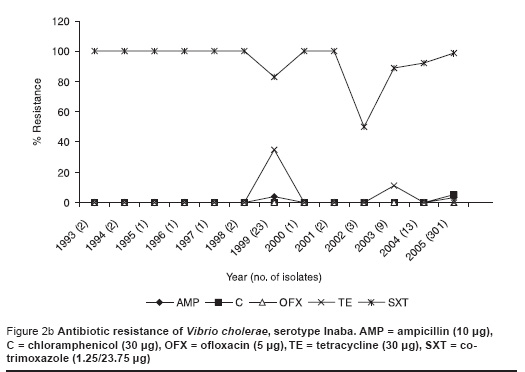
This recent VCI strain was 100% resistant to cotrimoxazole, 3% resistant to chloramphenicol and not resistant to ampicillin, tetracycline and ofloxacin (Figure 2a). This susceptibility pattern was very similar to the previous predominant serotype Ogawa (Figure 2b).
Discussion
This is the first report of increased isolation of serotype Inaba from Pakisatan. We have evaluated the data of Aga Khan University laboratory for the past 13 years and this increased pattern of VCI was never seen before. In a previous report by Jabeen et al., apart from predominant serotype Ogawa, there were increased numbers of cholera cases related to O139 serotype during the years 1993–1994 and 2000–2003 [3]. However, serotype Ogawa was never overtaken by any other serotype as it was in 2005 by VCI.
Two recent communications from India have reported the predominance of VCI in their cholera strains. One report was from Delhi in which 52% VCI were isolated in the year 2004, outnumbering VCO, the predominant strain in the past [6]. Taneja et al. from Chandigarh have reported the emergence of VCI in north India from a border security force camp [7].
In previous years, the isolation of VCI was rare and the most recent predominant outbreak was observed in 1989 in Calcutta [10]. Later, there were sporadic reports of outbreaks of VCI; one was from Malawi in 1990 which affected Mozambican refugees causing 1931 cases [11], another outbreak was in 1991 in which 8 patients in New Jersey, United States of America, developed cholera due to VCI after eating crabmeat [12]. Another outbreak of 12 cholera cases caused by VCI was reported from Hong Kong in 1994, which was linked with consumption of seafood [13]. A report of an outbreak from southern India in 1996 reported 13 nontoxigenic strains of VCI isolated from patients [14]. However, none of the above reports suggested continuous transmission, as in our study.
In 1984, there was a reported outbreak of nosocomial cholera involving 11 cases of VCI in southern Thailand [15]. A history of receiving tube-fed liquid diets was significantly more common among cholera cases than in matched controls. Cases were also significantly more likely than controls to be on oral antacid medication, which could increase the risk of infection by neutralizing gastric acidity. In our hospital, however, we have not seen any case of nosocomial cholera.
The susceptibility pattern of our recent VCI isolates is more-or-less similar to the previously predominant serotype Ogawa. Increased resistance to cotrimoxazole suggests the possibility of the presence of the SXT element, which is a self-transmissible, chromosomally integrated genetic element which carries cross-resistance to sulfamethoxazole, trimethoprim, streptomycin and furazoloidine [16]. However, we did not test the latter 2 antibiotics in our study. Another study by Garg et al. also reported that the antibiogram and pulsed-field gel electrophoresis pattern of their current VCI strains were similar to that of the prevailing VCO strains, suggesting seroconversion due to mutation in the wbeT region [17]. We suggest that there has been a serotype switching between the prevailing VCO strain and the current VCI strain due to mutation in the wbeT region, possibly because of immune pressure in the Pakistani population. However, we have not confirmed this fact as we have not performed molecular analysis of our VCI isolates. Quinolone resistance in V. cholerae has never been reported from Pakistan and 100% of our cholera isolates were sensitive to ofloxacin. However Das et al. from Delhi, India have reported that 18% of their VCI isolates were resistant to ciprofloxacin [6].
The mean age of both VCI and VCO patients was similar, involving a younger age group. This again suggests serotype conversion as the same age group is affected in both cases. This is in contrast to the outbreaks of VCO139 in Pakistan in 1993–94 and 2000–01 in which an older age group was affected [3].
In conclusion, we are reporting for the first time increased isolation of VCI from Pakistan and suggest that these VCI strains are wbeT mutants from the previously predominant VCO and presumably have arisen as a result of selection due to the immune response against VCO in the Pakistani population. However, molecular analysis is needed, as done by Garg et al. in Calcutta [17], to confirm our hypothesis. The effective transmission of this strain in the community suggests a decreased immune response in the population against this mutant VCI strain. Government intervention and active health education campaigns on water and food safety and personal hygiene are immediately required to reduce the risk of a major public health disaster as this strain has been involved in many outbreaks of cholera in recent months.
References
- Sack AD et al. Cholera. Lancet, 2004, 363:223–33.
- Colwell RR et al. Serogroup conversion of Vibrio cholerae. Canadian journal of microbiology, 1995, 41:946–50.
- Jabeen K, Hasan R. Re-emergence of Vibrio cholerae O139 in Pakistan: report from a tertiary care hospital. Journal of the Pakistan Medical Association, 2003, 53:335–58.
- Alam M et al. Seasonal variation in bacterial pathogens isolated from stool samples in Karachi, Pakistan. Journal of the Pakistan Medical Association, 2003, 53:125–9.
- Nizami S, Farooqui B. Cholera in children in Karachi from 1990 through 1995: a study of cases admitted to a tertiary care hospital. Journal of the Pakistan Medical Association, 1998, 48:171–3.
- Das S et al. Fluroquinolone resistance in Vibrio cholerae O1: emergence of El Tor Inaba. Annals of tropical paediatrics, 2005, 25:211–2.
- Taneja N et al. Emergence of Vibrio cholerae O1 biotype El Tor serotype Inaba in north India. Japanese journal of infectious diseases, 2005, 58:238–40.
- Koneman EW et al. Color atlas and text book of diagnostic microbiology, 5th ed. Philadelphia, Lippincott, 1997.
- Performance standards for antimicrobial susceptibility testing. 15th informational supplement. Volume 25(1). Wayne, Pennsylvania, Clinical and Laboratory Standards Institute, 2005.
- Ramamurthy T et al. Serovar, biotype, phage type, toxigenicity and antibiotic susceptibility patterns of Vibrio cholerae isolated during two consecutive cholera seasons (1989-90) in Calcutta. Indian journal of medical research, 1992, 95:125–9.
- Swerdlow DL et al. Epidemic cholera among refugees in Malawi, Africa: treatment and transmission. Epidemiology and infection, 1997, 118:207–14.
- Finelli L et al. Outbreak of cholera associated with crab brought from an area with epidemic disease. Journal of infectious diseases, 1992, 166:1433–5.
- Kam KM et al. Outbreak of Vibrio cholerae 01 in Hong Kong related to contaminated fish tank water. Public health, 1995, 109:389–95.
- Saha PK et al. Nontoxigenic Vibrio cholerae 01 serotype Inaba biotype El Tor associated with a cluster of cases of cholera in southern India. Journal of clinical microbiology, 1996; 34:1114–7.
- Swaddiwudhipong W, Kunasol P. An outbreak of nosocomial cholera in a 755-bed hospital. Transactions of the Royal Society of Tropical Medicine and Hygiene, 1989, 83:279–81.
- Waldor MK, Tschäpe H, Mekalanos JJ. A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim and streptomycin in Vibrio cholerae O139. Journal of bacteriology, 1996, 178:4157–65.
- Garg P et al. Emergence of Vibrio cholerae O1 biotype El Tor serotype Inaba from the prevailing O1 Ogawa serotype strains in India. Journal of clinical microbiology


