A. Munim,1 Y. Rajab, 1 A. Barker,2 M. Daniel2 and B. Williams3
اختطار العدوى بالمتفطِّرات السلية في الصومال: المسح الوطني للتوبركولين عام 2006
عايد منعم، يوسف رجب، آنا باركر، ماري دانيال، براين وليم
الخلاصـة: أجري المسح الوطني للتوبركولين في الصومال في ما بين شباط/فبراير وآذار/مارس 2006 لتقدير الاختطار السنوي للعدوى بالسل. وأخذت عينات من مجموعات طبقاتية في 18 منطقة و101 مدرسة ابتدائية تم اختيارها عشوائياً. وأجري اختبار التوبركولين لدى 686 10 من طلاب المدارس في المرحلة الأولى، مع قياس مساحة التفاعل بعد 72 ساعة. وكان عدد الأطفال الذين لديهم قراءة مقبولة 364 10 طفلاً. وكان معدل الإجمالي للتغطية بلقاح بي سي جي 54%، ويقدر أن الاختطار السنوي للعدوى بالسل في الصومال يبلغ 2.2% استناداً على تكرار توزَّع مساحات تفاعل التوبركولين (فاصلة الثقة 1.5% - 3.2%). وقد حدث انخفاض سنوي مقداره 2.6% بالمقارنةً مع الدراسة التي أجريت سابقاً عام 1956.
ABSTRACT: To estimate the annual risk of tuberculosis (TB) infection (ARTI) in Somalia a tuberculin survey was conducted in February/March 2006. Stratified cluster sampling was carried out within the 18 regions and 101 randomly selected primary schools. Tuberculin testing was done in 10 680 grade 1 schoolchildren. Transverse tuberculin reaction size was measured 72 hours later. The number of children with a satisfactory test read was 10 364. The overall BCG coverage was 54%. Based on frequency distribution of tuberculin reaction sizes, the ARTI in Somalia was estimated at 2.2% (confidence interval: 1.5%–3.2%). There was an annual decline of 2.6% comparing with a previous study in 1956.
Risque d’infection à Mycobacterium tuberculosis en Somalie : enquête tuberculinique nationale 2006
RÉSUMÉ: Afin d’estimer le risque annuel d’infection tuberculeuse en Somalie, une enquête tuberculinique a été réalisée en février-mars 2006. Un échantillonnage en grappes stratifié a été effectué dans les 18 régions et dans 101 écoles primaires choisies au hasard. Un test tuberculinique a été pratiqué sur 10 680 écoliers de première année. Le diamètre de la réaction tuberculinique a été mesuré 72 heures plus tard. Le nombre d’enfants pour lesquels la lecture du test était satisfaisante était de 10 364. La couverture globale par le BCG était de 54 %. D’après la distribution de fréquence du diamètre de la réaction tuberculinique, le risque annuel d’infection tuberculeuse en Somalie a été estimé à 2,2 % (intervalle de confiance : 1,5 % - 3,2 %). Il y a eu une baisse annuelle de 2,6 % par rapport à une étude antérieure de 1956.
1World Health Organization, Hargeisa, Somalia (Correspondence to A. Munim:
2Desmond Tutu Tuberculosis Centre, University of Stellenbosch, Cape Town, South Africa.
3World Health Organization, Geneva, Switzerland.
Received: 31/01/07; accepted: 25/06/07
EMHJ, 2008, 14(3):518-530
Introduction
Somalia is a country in a complex emergency situation due to the long-lasting civil conflict and natural disasters. Somalia had an overall human development index (HDI) of 0.299 in 2002, which places the country among the 5 least developed countries in the world according to United Nations Development Programme (UNDP)/World Bank Socioeconomic Survey 2002 Somalia [1]. The extensive movement of people in Somalia, the low literacy rate and widespread poverty have left the country in a precarious state.
In the past Somalia has had an exceptionally high incidence and prevalence of pulmonary tuberculosis (TB) [2,3]. In a survey carried out by the World Health Organization (WHO) in 1955 and 1956 among children aged 8 to 12 years, the annual risk of TB infection (ARTI) was estimated at 8% [2]. In a survey carried out among Somali refugees in the Ogaden region of Ethiopia in 1984 the ARTI was 4.8%, [4] and in a Finnish project carried out in 1986 in Burao, Somaliland, and Kismayo, South and Central zone, the ARTI at the age of 10 years was 3.7% (3.3%–4.1%) per year (here and elsewhere intervals given are 95% confidence intervals) with a cut-off at 5 mm and 2.9% (2.5%−3.3%) per year with a cut-off at 10 mm [5]. From the 1986 survey, and assuming a Stýblo constant of 50 (40−60), the smear-positive incidence was then 185 (143−228) per 100 000 population per year using a cut-off at 5 mm, or 145 (105−180) per 100 000 population per year using a cut-off at 10 mm. The TB programme in Somalia currently assumes that the incidence is 162/100 000 population per year for smear-positive cases and 324/100 000 population per year for all forms of TB [6]. According to the United Nations Population Division, the population of Somalia was 6.3 million in 1995 and 8.2 million in 2005 [7]. Over this period, smear-positive notifications increased from 1572 to 7068 [8] so that the notification rate increased from 25/100 000 population to 86/100 000 population and the smear-positive case-detection rate increased from 15% (12%−18%) to 53% (43%−63%).
Because of the extent of the TB burden in the community, all Somalia Aid Coordination Body (SACB) health partners, including health authorities, WHO, World Food Programme, and international nongovernmental organizations (NGOs) have given priority to TB control. In 2003 a joint proposal was developed by SACB under the auspices of the Health Sector Committee and approved by the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM). Part of this proposal required that a tuberculin survey be carried out at year 1 of its implementation so as to asses the ARTI (and to adjust the programme targets accordingly) and to be repeated at year 5 so as to asses the impact of the TB control activities.
The Somalia TB Programme started implementing directly observed treatment, short-course (DOTS) in 1995, and achieved the regional targets of DOTS all over, based on the presence of at least 1 TB centre in each of the 18 regions of Somalia. However, the presence of vast regions where the people are nomadic combined with the security problems related to the protracted political crisis contribute to the inaccessibility of some of these centres. The expansion of the TB centres, so that there is more than 1 centre in the larger regions and towns, is expected to contribute to improving the case-detection rate in places where detection is low. The treatment outcomes are good. In 2005, 58% of cases were smear-positive, 24% were smear-negative, 17% were extra-pulmonary and 4% were relapse cases. The treatment success rate was 90% with 2% dying, 1% failing treatment and 4% defaulting.
Tuberculin skin test surveys can be used to assess the size of the TB problem as well as to determine trends over time provided it is possible to collect good data. In addition tuberculin skin test surveys can provide important information that helps us to understand the epidemiology of TB [9–12].
In order to assess the current levels of TB in Somalia, the trends in the prevalence of TB infection since the earlier surveys, and the progress towards the Millennium Development Goals for TB, a national survey of TB infection was carried out in late February and early March 2006 by WHO Somalia in collaboration with the Ministry of Health, and the Expanded Programme on Immunization, the women’s federation in each zone, and all partners from the SACB TB working group. This survey was sponsored by GFATM through World Vision (principal recipient).
The objectives of the study were to:
Determine the ARTI. This will be used as baseline for re-setting the programme targets and to evaluate the impact after 5 years.
Determine the trend in TB infection by comparing the current ARTI with the results of previous surveys.
Determine BCG scar coverage among a cohort of Somali first-grade primary-school students in Somalia.
Methods
Organization of the survey
First-grade primary-school pupils for the academic year 2006–2007 were the target population for this survey. Somalia is in a protracted and complex emergency situation and for the purposes of the survey the country was divided into management teams for each of 3 zones (South and Central Zone, Puntland and Somaliland) and each team was supported by qualified supervisory teams according to the sample size and operating scenarios. All supervisory teams were instructed as trainers of trainees (TOT) [12]
The survey was done using stratified cluster sampling within the 18 regions of the country; 32 districts within these regions and 101 schools out of 1172 operating schools within these districts according to the UNICEF Survey of Primary Schools in Somalia for 2003–2004 were selected. Thirty (30) teams, each consisting of 2 people, and 15 supervisors were employed to carry out the testing and to read the indurations. The teams were trained by internationally recognized trainers in February, 2006, and a pilot project was carried out in Hargeisa in order to validate the test and the training of the staff.
The antigen used was purified protein derivate (PPD) RT-23 tuberculin Tween 80 (vial of 5 mL, batch number 1461K obtained directly from the Statens Serum Institute, Copenhagen, Denmark). Tuberculin was transferred to Somalia by air and immediately stored in a temperature controlled refrigerator at WHO warehouses in Hargeisa, Mogadishu and Garowe, from which only the required number of vials were made available for each survey day. Vials were transported in cold boxes packed with ice and once opened were used within a few hours to minimize decay of the active substance.
The Mantoux technique was used with disposable 1 mL tuberculin syringes and 26 gauge needles. PPD antigen (2 tuberculin units as recommended by WHO, in a volume of 0.1 mL) was injected intradermally into the volar surface of left forearm [9,11,13]. The induration was measured transverse to the long axis of the arm after 72 hours following international recommendations [2]. If the injection was given too deeply or a loss of tuberculin occurred, it was repeated on the right arm.
In addition to the children included in the main survey, 14 adults sputum smear-positive tuberculosis patients were tested to assess the expected mode in the distribution of reaction sizes [2]. A special form was used for data collection and data were entered using Epi-Info, version 3.2.
Ethical considerations
Verbal informed consent was obtained from the parents of the children and an official letter was circulated from the Ministries of Education and Health to all schools involved in the survey. We explained to them the procedure and the objectives of the test. This procedure was organized by members of the tuberculin survey committee in each zone in collaboration with local community elders.
Data entry and cleaning
The data were entered by 2 trained data entry staff and each record was double-checked individually by 4 teams of people. When the data had been checked, the entire data set was checked for consistency. SPSS, version 10.0 was used to make the final check on consistency.
We started by examining the proportion of responses that were greater than zero for each of the testers as shown in Figure 1. We could be confident that the annual risk of infection is between 1% and 10% so that the lowest point could be excluded and the 12 highest points could be excluded. We also examined the distribution of induration measurements for each tester and excluded 6 testers for which the distribution was significantly different from the overall average. Because the 2 categories overlapped only 15 readers were excluded. The age range was 3 years to 37 years old but since 93% of those tested were between 6−12 years, we retained only those 6−12 years old inclusive.
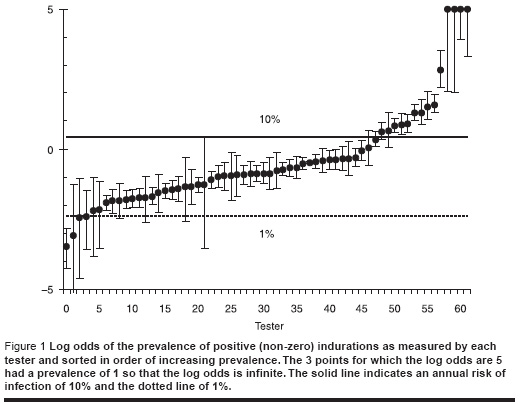
Results
A total of 10 680 people were tested; absent students and refusals were excluded. In the entire data set only 28 ages, 52 readings of the BCG and 316 readings of the induration were missing and these were excluded; thus 10 364 indurations were read.
Of the total number sampled, 59% were male and 41% were female. The mean ages of males and females in the sample were 9.3 years (interquartile range: 8 to 11 years) and 9.2 years (interquartile range: 8 to 11 years) respectively.
Annual rate of tuberculosis infection
The ARI of infection for Somalia was estimated as follows. For the data shown in Figure 1 the proportion of positive responders was 0.30 (0.29−0.31) From the fit in Figure 1 the proportion of these that were due to Mycobacterium tuberculosis was 0.61 (0.17−0.85) so that the prevalence of M. tuberculosis infection was 0.18% (0.05−0.26). The average age of those tested and not excluded was 9.09 years giving an annual rate of decline of 2.22% (0.58%−3.18%).
The 1986 study followed the recommendations of the WHO in which all indurations of 10 mm or more were taken as indicating M. tuberculosis infection [12]. If we apply this criterion to the present survey, the prevalence of M. tuberculosis infection is 2.21%, very close to the value estimated above.
Fitting the data
The distribution of induration measurements, after excluding the readers as noted above, are shown by the blue dots in Figure 2. There was significant digit preference, especially at 10 mm, and no clear indication of 2 separate underlying distributions corresponding to the response to environmental mycobacterium and to TB.
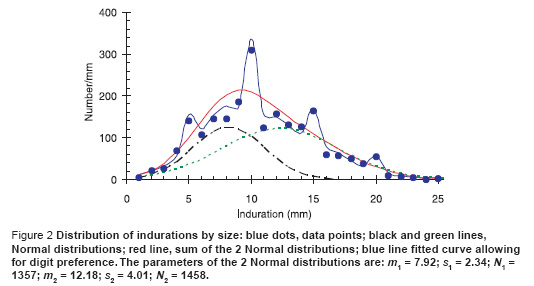
We were guided by the data from the 1956 tuberculin survey in Somalia [2] that showed 2 clear peaks at 5 mm and at 15 mm each of which approximates a normal distribution function in shape. We therefore proceeded as follows. We started from 2 Normal distribution functions whose sum will give the total distribution. To allow for digit preference, we then added a further 8 parameters which gave the probability that a point at 3 or 7 mm is misreported as 5 mm, that 4 or 6 mm is misreported as 5 mm, and then did the same for the points on either side of 10 mm, 15 mm, and 20 mm. While there are a total of 11 parameters, the last 8 do not change the area under the curve but only the distribution of the points around 5, 10, 15 and 20 mm. (A more sophisticated way of doing this has been explored by Eilers et al. [14]). The solid blue line is then the maximum likelihood fit to the data assuming Poisson statistics and the red line is the underlying sum of normal distributions. Assuming that the area under the green curve represents the response to M. tuberculosis and the area under the black curve represents the response to environmental bacteria we can then determine the proportion of all positive (non-zero) reactors that are due to M. tuberculosis. To determine confidence intervals on the estimated proportions we then find the maximum and minimum values of the area under the green curve for which the deviance differs from the minimum value by c 2n (0.05) where n is the number of free parameters [15] which we vary to minimize the deviance for the given area under the green curve.
Hospital patients
As a final check, 14 patients, hospitalized with TB, were given a tuberculin skin test and the size of the indurations was measured over the following 3 days; the results are shown in Table 1. The size of the induration did not vary significantly among the days or between the sexes, and the average induration size over all days and patients was 18.1 (1.69−19.3) mm. Assuming that the peak induration among people who are not TB patients is close to 18 mm, a cut-off in the region of 10 mm is not unreasonable. However, we note that the implied peak of the tuberculosis response in Figure 2 was at 12 mm, significantly lower than the value measured in the TB patients, which was 18 mm.
Dependence of responses on testing delay
The indurations were read 3 or 4 days after the tuberculin test was administered. The proportion of positive reactors when the tests were read on day 4 (36%; 33%−39%) was about 25% greater than the proportion when the tests were read on day 3 (29%; 27%−28%). This could introduce a further uncertainty of about 25% into the estimates of ARTI.
Dependence of response on age
Figure 3 shows the dependence of the proportion of positive reactors (scaled down by a factor of 0.61, the estimated proportion of positive reactors that are infected with tuberculosis) by age showing an increase with age which is more or less as expected assuming a constant annual risk of infection.
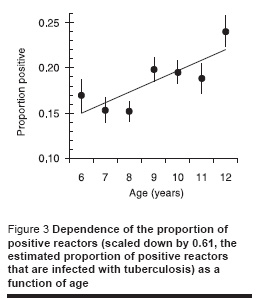
Dependence of ARTI on BCG scar
The overall mean proportion of positive reactors to tuberculin was 0.3%. The proportion of positive reactors was significantly lower in those with a BCG scar (0.283%; 0.270%−0.296%) than those without a BCG scar (0.326%; 0.309% − 0.343%). The proportion of positive reactors with uncertain BCG status was 0.302% (0.257%−0.347%).
In order to estimate the ARTI for those with and without a BCG scar we fitted the data separately for those with and without BCG scars as described above. However, we kept the mean (SD) of the 2 normal distributions fixed and varied only the normalization constants; the results are shown in Table 2 from which we can obtain an estimate of the odds ratio of TB infection for those with and without a BCG scar. The value obtained in this way is 1.37 (1.30−1.44) suggesting that BCG vaccination gives significant protection against TB infection. Both estimates of ARTI are of course uncertain (as indicated above) but provided the biases are in the same direction, the odds ratio will still differ significantly.
Prevalence of infection
Table 3 shows the prevalence of infection in males and females, in each of the 3 zones, and for urban and rural schools assuming that everyone with an induration of 10 mm or more is positive. There were more males than females in the sample but females were at significantly greater risk of infection than males. Nearly two-thirds of the sample were in the South and Central Zone with about a quarter in Puntland and just over one-tenth in Somaliland. There were statistically significant differences in TB infection and ARTI between the 3 zones with the highest prevalence and ARTI in South and Central zone and the lowest in Puntland. The ARTI in Puntland was only half that in South and Central zone. The sample was predominantly urban but there was no significant difference between the prevalence of infection in urban and rural areas.
Figure 4 shows the trend in the ARTI over time using data from the 3 surveys. Fitting an exponential trend line to the data shows that over the period from 1958 to 2006, the ARTI has fallen at a rate of about 2.6% per year over the last 50 years.
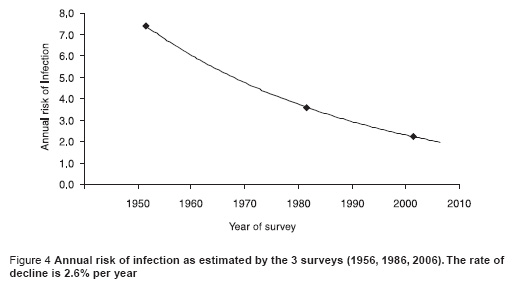
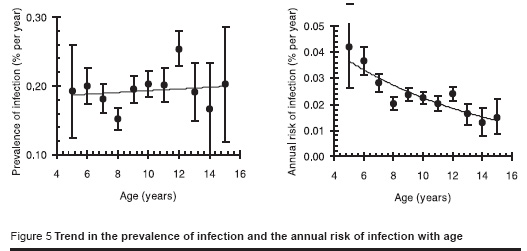
Figure 5 shows the trend in the prevalence of infection with age for males and females combined (the trend was not significantly different between males and females, P = 0.4). The data suggest that the ARTI, averaged over each person’s life time, is less for older than it is for younger people and that there is a declining annual risk of infection of about 10% per year of age.
BCG coverage
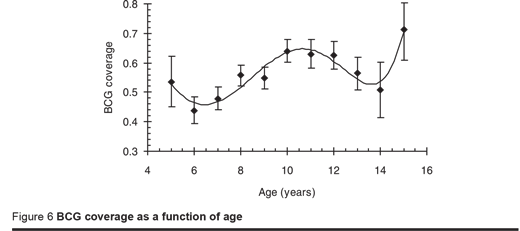
The overall BCG coverage was 54% with a further 5% recorded as doubtful. This is near to the EPI programme coverage reported by WHO/UNICEF in 2005, which was 60%. The coverage varied significantly by zone with the lowest coverage in Somaliland (38%) and significantly higher coverage in Puntland (62%) and South and Central zone (58%); coverage did not differ significantly between males (53%) and females (54%). Figure 6 gives the BCG coverage by age. Reading back then, the coverage of BCG appeared to fall 14 years ago, increased over the next 4 years but then fell again over the following 4 years. This finding matches the WHO/UNICEF report of 2005 which was 37% in 1992 and 37% in 1997. The prevalence of infection was no different between those with or without a BCG scar.
Estimates of the case-detection rate
Using the estimated values of the ARTI in Table 3 we used the Stýblo rule to estimate incidence and hence the case-detection rate. However, converting notification numbers to notification rates depends on the population. Using the current estimate of the total population from the United Nations Population Division noted above [7] and allowing for a population increase between 2005 and 2006 gives a total population of 8.65 million. In 2006 there were 7040 notified cases of TB in Somalia giving a notification rate of 81/100 000 population per year (Table 4). The ARTI gives an estimated incidence of 111/100 000 population per year (75−160/100 000 population per year) or a case-detection rate of 74% (51%−108%).
Discussion
Because of the prolonged civil war and the complex situation in Somalia, it has been difficult to obtain reliable data about the actual incidence and prevalence of TB. Nevertheless, if the prevalence of infection with TB can be successfully estimated and repeat surveys are available, it should be possible to draw some conclusions about the transmission in the community and thus the development of the epidemiology of TB over time. Two surveys were previously conducted in Somalia, one in 1956 and the other in 1986 [3,5], in addition to a survey among Somali refugees in the Ogaden region of Ethiopia in 1984 in which the ARTI was 4.8% [4].
The participation rate was very high with only 3% of people who were tested not having a successful reading of their induration. By way of comparison, in a recent survey in Malawi, 21% of those tested did not have a successful reading of their induration [16].
The best estimate of the national ARTI in Somalia is 2.2% per year (1.5%−3.2% per year). Comparing this with the results of earlier surveys suggests an average annual rate of decline in the prevalence of infection between 1956 and 2006 of about 2.6% per year. If the present rate of decline in the prevalence of infection is maintained, then between 1990 and 2015 the prevalence will fall by 46%. The decline in ARTI over time is similar to the decline found in surveys carried out in Kenya, Ethiopia and Egypt [17–19].
There was significant variation in the ARTI in the 3 zones of the country with the highest rates in the South and Central zone. In the country as a whole BCG coverage was 54% but it was highest in Puntland (62%), then in South and Central zone (58%), and lowest in Somaliland (38%). There was no significant difference in the prevalence of infection between males and females or urban and rural areas.
There are some important caveats that need to be taken into account when considering our results. First, the testing of TB patients suggests that the mode of the distribution should be at 18 mm but in fact only 2.2% of indurations exceed 18 mm so that the corresponding ARTI would only be 0.5% per year using a mirror method. If, on the other hand, we were to assume that all non-zero indurations were due to TB, then the prevalence would be 38% and the ARTI 5.1%, which would roughly halve the case-detection rate. It is important to understand the reasons for the low peak and to decide definitively how best to apportion the positive indurations to TB infection. Second, the estimate of the notification rate per 100 000 population and hence the case-detection rate depends on the population size, which in Somalia is very uncertain given the social and political uncertainty in the country. Third, there was a variance in grade 1 enrolment between boys and girls (61% for boys and 39% for girls according to a UNICEF survey of primary schools in Somalia 2004) and finally there was a refusal in one region which included about 5% of the sample size.
Recommendations
A further survey should be carried out in 5 years and again in 10 years to help monitor progress towards the Millennium Development Goals.
Further analysis should be done to explore the variation in the distribution of indurations spatially as well as by age, tester and BCG status in order to understand the determinants of the distribution.
Acknowledgements
Carrying out a survey of this kind in a complex emergency setting like Somalia was a daunting task. Many people provided substantial support and the authors would like to acknowledge in particular the Somali health authorities and the members of the tuberculin survey committees, Dr Ismail Adan, Dr Abukar Ali Hilowle, Dr Bashir Suleiman and Dr Ali Hassan. Special thanks go to Dr Imanol Berakoetxea for his support during preparation and implementation. We also extend our gratitude to all testers and readers for their distinguished efforts despite the harsh and difficult situation, and to all members of the TB working group, especially CCM Italy teams (Mr Peter Wilson, Dr Pardeep Kapoor, Dr Harun I. Hassan, Dr Said Adan, Mr Ismail Arte Rage, Mrs Ahadha Abdi and Mr Abdirahman Ibrahim), and to all TB partners, Ministry of Health, Ministry of Education, Women’s Federation and elders for their support and authorization to carry out the survey. We would also like to thank Dr Ibrahim Betelmal, WHO Representative in Somalia, and his team for continuous support and leadership; also Dr Akihiro Seita, Dr Samiha Baghdadi, Dr Zoheir Hallaj, Dr Hans Reider and Dr Christopher Dye for their guidance and support.
This survey was made possible through funding provided by the Global Fund through the principle recipient World Vision, but especially Dr Vianney Rusagara and his team.
References
- United Nations Development Programme (UNDP)/World Bank. Socioeconomic Survey 2002 Somalia (http://mirror.undp.org/somalia/docs/whole%20SWB%20book.pdf, accessed 21 January 2008).
- Tuberculosis survey in the Somalilands. Copenhagen, World Health Organization Regional Office for Europe, 1956.
- Young K. Impressions of tuberculosis in Somaliland. Tubercle, 1957, 38:273–9.
- Shears P. Tuberculosis control in Somali refugee camps. Tubercle, 1984, 65:111–6.
- Peltola H et al. Risk of infection with Mycobacterium tuberculosis among children and mothers in Somalia. Clinical infectious diseases, 1994, 18:106–11.
- Global tuberculosis control: surveillance, planning, financing. WHO report 2007 . Geneva, World Health Organization, 2007 (WHO/HTM/TB/2007.376).
- World migrant stock: The 2005 revision population database. New York, United Nations Population Division, 2006.
- Munim A. Summarized progress report on WHO supported TB control programme in Somalia 2005. Somalia, World Health Organization Somalia, 2005.
- Styblo K. The relationship between the risk of tuberculous infection and the risk of developing infectious tuberculosis. Tubercle and lung disease, 1985, 60(3–4):117–9.
- Palmer CE et al. Experimental and epidemiologic basis for the interpretation of tuberculin sensitivity. Journal of pediatrics, 1959, 55:413–29.
- Wijsmuller G et al. On the nature of tuberculin sensitivity in South India. American review of respiratory disease, 1968, 97:429–43.
- The standard tuberculin test. Geneva, World Health Organization, 1963 (WHO Technical Guide, 3).
- Arnadottir T et al. Guidelines for conducting tuberculin skin test surveys in high prevalence countries. Tubercle and lung disease, 1996, 77(Suppl. 1):1–19.
- Eilers PH, Borgdorff MW. Modelling and correction of digit preference in tuberculin surveys. International journal of tuberculosis and lung disease, 2004, 8:232–9.
- Williams B et al. Estimating HIV incidence rates from age prevalence data in epidemic situations. Statistics in medicine, 2001, 20:2003–16.
- Salaniponi FM et al. Risk of infection with Mycobacterium tuberculosis in Malawi: national tuberculin survey 1994. International journal of tuberculosis and lung disease, 2004, 8:718–23.
- Bosman MC et al. National tuberculin survey of Kenya, 1986–1990. International journal of tuberculosis and lung disease, 1998, 2:272–80.
- Azbite M. National tuberculin test survey in Ethiopia. Ethiopian medical journal, 1992, 30:215–24.
- El Ibiary S et al. Trend in the annual risk of tuberculous infection in Egypt, 1950–1996. International journal of tuberculosis and lung disease, 1999, 3:294–9.


