H. Vatandoost1 and A.A. Hanafi-Bojd1
تقييم مختبري لثلاثة طاردات تستخدم لطرد الأنوفيلة الاصطفانية في جمهورية إيران الإسلامية
حسن وطن دوست، أحمد علي حنفي بُجْد
الخلاصـة: أجريت هذه الدراسة لتقييم التأثير التنفيري لثلاثة طاردات موضعية للناموس وهي(البريمثرين، وثنائي إيثيل طولواميد DEET)، وخلاصة شجر النيم) ضد إناث ذراري الأنوفيلة الاصطفانية، المختبرية والحقلية التي تـتـراوح أعمارها ما بين ثلاثة وخمسة أيام. وقد تم قياس معدلات السَّبْر أو اللدغ التي تواجدت على البطون المحلوقة الشعر للأرانب البيضاء، وتم حساب القيم ED50 وED95 باستخدام الوسائل الحاسوبية للإحصاء. وقد كانت القيم التي استخلصت هيED50، 0.007، و0.191 مغ/سم2 لكل من البريمثرين، وثنائي إيثيل طولواميد والنيم علـى التوالـي، ضـد الذراري الحقليـة. أمـا النسب المقابلـة لذراري المختبـرات فكـانت 0.006، و0.007، و0.156 مغ/سم2. وقد لوحظ أن التغايُرية الرئيسية للاستجابة كانت عند استخدام ثنائي إيثيل طولواميد DEET. وعلى الرغم من أن شجر النيم كان أقل العوامل فعّاليةً، إلا أن خلاصات النيم المنتجة محلياً تنبئ عن استنباط طارد واعد جداً ضد لدغات البعوض.
ABSTRACT: This study evaluated the repellency effect of 3 topical repellents (permethrin, DEET and neem tree extract) against 3–5 day old females of laboratory and field strains of Anopheles stephensi. Probing/biting rates on the shaved belly of white rabbits were counted. Effective dose (ED) 50 and ED95 values were calculated by probit statistic software. The results revealed ED50 values of 0.007, 0.005 and 0.191 mg/cm2 for permethrin, DEET and neem, respectively, against the field strain. The figures for the laboratory strain were 0.006, 0.007, 0.156 mg/cm2. Major heterogeneity of response was observed using DEET. Although neem was the least effective agent, extracts of locally produced neem oil offer a promising repellent against mosquito biting.
Évaluation en laboratoire de trois répulsifs contre Anopheles stephensi en République islamique d’Iran
RÉSUMÉ: Cette étude a évalué les effets de trois répulsifs topiques (perméthrine, DEET et extrait de margousier) contre des femelles de souches de laboratoire et de terrain d’Anopheles stephensi âgées de 3 à 5 jours. Les taux de piqûres/probing (pénétration des pièces buccales du moustique) sur le ventre rasé de lapins blancs ont été comptés. Les valeurs ED50 et ED95 (ED pour effective dose : dose efficace) ont été calculées à partir d’un logiciel statistique fondé sur le modèle probit. On a ainsi obtenu des valeurs ED50 de 0,007, 0,005 et 0,191 mg/cm2, respectivement, pour la perméthrine, le DEET et le margousier, par rapport à la souche de terrain. Pour la souche de laboratoire, ces chiffres étaient de 0,006, 0,007 et 0,156 mg/cm2. Une hétérogénéité de réponse très importante a été observée avec le DEET. Bien que le margousier ait été l’agent le moins efficace, les extraits d’huile de margousier produits sur place constituaient un répulsif prometteur contre les piqûres de moustiques.
1Department of Medical Entomology and Vector Control, School of Public Health and Institute of Health Research, Tehran University of Medical Sciences, Tehran, Islamic Republic of Iran (Correspondence to H. Vatandoost:
Received: 31/03/05; accepted: 08/02/06
EMHJ, 2008, 14(2): 260-267
Introduction
Over 2 billion people, primarily in tropical countries, are at risk from mosquito-borne diseases, such as dengue haemorrhagic fever, malaria and filariasis [1]. In the Islamic Republic of Iran arthropod-borne diseases such as malaria and leishmaniasis are the main public health problems. The key weapons against these are insecticides. However, the widespread use and toxicological profile of insecticides, as well as increasing insecticide resistance, is often problematic.
The use of topical repellents to prevent arthropod bites is an effective personal protection measure to reduce or prevent transmission of these diseases. Insect repellents may be as economical as vector control operations and are an alternative to chemical vector control [2].
This study tested the effectiveness of 3 repellants:
N,N-diethyl-3-methylbenzamide (DEET) is an effective broad-spectrum repellent, and is the main ingredient in many topical repellents currently available for use against insects and other arthropods affecting humans [3].
The neem tree, Azadirachta indica A. Juss (Meliaceae), is known for its insecticidal properties [4] and the alkaloids of the neem tree have been investigated as insect antifeedants [5]. Indian scientists evaluated the efficacy of this compound as a repellent against mosquitoes and sandflies [6,7].
Permethrin [(3-phenoxyphenyl)methyl(±)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethyl cyclopropane carboxylate] is a synthetic pyrethroid. It is used in commercial creams and is applied to fabrics for personal protection against mosquitoes [8–12].
Field and laboratory tests with anopheline mosquitoes have shown a wide range of sensitivity to repellents in different species as well as different areas. A comparison of 3 repellent products against Aedes aegypti and Anopheles stephensi showed that An. stephensi were equally sensitivity to the tested repellents while Ae. aegypti showed tolerance to 1 compound. This provided evidence that the repellent receptor systems of the species are physically different [13]. It suggests that different species of arthropods, strains within species, and individuals within strains, can vary in their susceptibility to repellent compounds. This premise is supported by Rutledge et al. [14], who observed that 18 mosquito species and strains displayed significantly different levels of susceptibility to the repellent effects of DEET [10,15–17]. Therefore, it is necessary to establish the sensitivity of individual species in every malarious area.
An. stephensi Liston is the main malaria vector in southern Islamic Republic of Iran. It is resistant to dichloro-diphenyl-trichloro-ethane (DDT), dieldrin and malathion in this area [18,19]. This study was designed to evaluate 3 repellents against laboratory- and field-collected strains of An. stephensi under laboratory conditions.
Methods
Mosquitoes
An. stephensi strains used in the tests were obtained from the laboratory as well as collected from the field. The colonies were maintained in Bandar Abbas Training and Public Health Research Centre. They were reared under insectary conditions at 25–29 ºC, 12:12 (light:dark) hour photo-period and 50%–70% relative humidity, and were fed with 10% aqueous sucrose solution. The field strain was reared for 5 generations before testing, while the insectary strain was kept for more than 4 years. Starved 3–5-day-old females were used for the tests. The sucrose solution was withdrawn from the cage 14 hours prior to the tests.
Repellents
The following technical-grade chemicals were tested: DEET was purchased from Merck, Germany (8.17033.1000 diethyltoluamide USP, batch S36954, assay 98.8%, density 0.998 g/L). Neem extract was provided from fruit extraction of local plants (grown in different parts of Bandar Abbas) by the Faculty of Pharmacology, Tehran University of Medical Sciences. The fruits were kept in ethanol for 1 week, filtered and concentrated under vacuum. Six phytochemical specific tests used on the extracts. Gas chromatography/mass spectrometry analysis showed the presence of alkaloids, acetogenin, tannins and triterpenoids and unsaturated oils. Permethrin was provided by Bayer, Germany.
Test method
White rabbits were used to determine the effective doses (EDs) of repellents. The test used a modification of the Klun and Debboun module [20]. A modified ASTM [American Society for Testing and Materials] standard plastic cage (1983) was employed [3]. The internal walls of the apparatus were removed to create a single cell with the lower surface lined with cotton net. The ED tests were conducted by applying each repellent directly to the shaved belly of the rabbit. For each dose only 1 rabbit was used. To prevent interference only 1 dose of repellent was applied in each test. For the control experiment only ethanol solvent was applied. The treated areas were allowed to dry and then the test chamber containing mosquitoes was fixed onto the treated shaved belly. Then 10–15 mosquitoes were released into the test apparatus. Probing/biting counts were recorded at 1 minute intervals up to 5 minutes. Each test cage was used only once for a given dose. After every test, mosquitoes were removed from the test chamber using an aspirator and then transferred into a sleeved-screened cage.
Tests were repeated on different day intervals in order to obtain an estimate of ED50 and ED95. The concentrations used depended on the repellent type; the lowest and highest concentrations of repellents used were 0.0005 mg/cm2 and 1.2 mg/cm2 respectively.
Statistical analysis
The cumulative results were subjected to statistical analysis. Dosage–biting regression lines were determined by probit analysis using a special computer programme. Goodness-of-fit of the points to a straight line were tested by the chi-squared test. Data were computer analysed by the probit plane procedure using MicroProbit, version 3.0 software. The analysis for each test yielded ED50, ED95, confidence interval (CI) and slope values. Significant differences were determined by comparing the ED50s and 95% CI. The heterogeneity of the population was determined by the chi-squared test. The regression line was plotted using Microsoft Excel.
Results
The ED50 value (median effective dose) for permethrin against the laboratory strain of An. stephensi was 0.006 mg/cm2 (Table 1). The figures for DEET and neem were 0.007 and 0.156 mg/cm2 respectively. The probit regression line is plotted in Figure 1 and the slope values for each repellent are presented in Tables 1 and 2. These values for the laboratory strain were –0.73, –1.19 and –1.52 for permethrin, DEET and neem respectively. Similar values were obtained with the field strain: –0.75, –1.20 and –1.32 for permethrin, DEET and neem respectively.
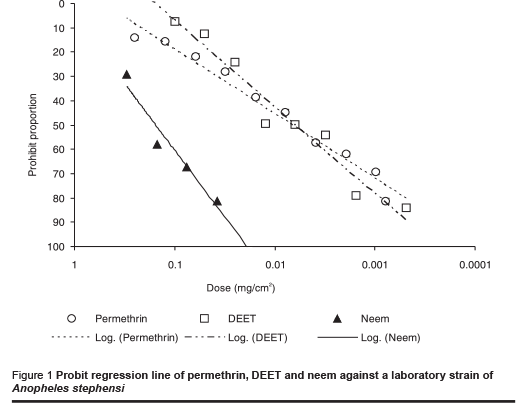
The results of tests against the field-collected strain of An. stephensi are shown in Table 2 and the probit regression line in Figure 2. The order of repellency effect of the 3 repellents against field strains of An. stephensi was DEET (strongest), permethrin and neem (weakest).
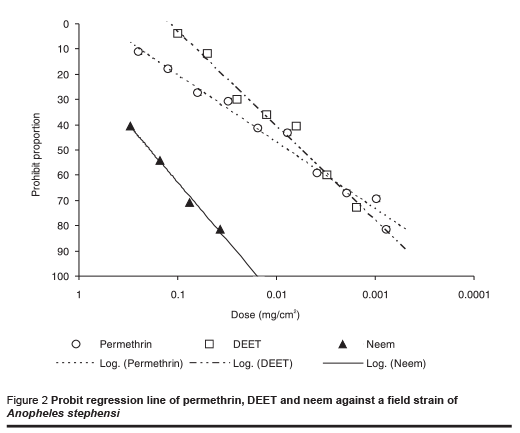
In the control tests almost all the starved mosquitoes fed during the test period. Although data from repellent studies using different test methods are not directly comparable as different workers have shown [21], the relative sensitivity of different strains of An. stephensi to a particular repellent can be compared. At the ED50 level, different repellents showed no significantly different effect on laboratory and field strains, but the amount of neem required was much higher than the other 2 repellents to cause the same response in both strains of mosquito (P < 0.001).
Discussion
In a comparative study of 4 Anopheles strains to 5 repellents, the effectiveness of permethrin was second to DEET [10]. The researchers found probit log-dose slopes were consistently the lowest for permethrin, but our results showed a slope of –0.73 for permethrin and –1.19 for DEET in the laboratory strain. In their study, the ED50 value for permethrin was 0.0026 (95% CI: 0.002–0.0033) and ED95 was 0.0156 (95% CI: 0.0097–0.0303) mg/cm2. Studies on the repellent effect of DEET on An. stephensi showed an ED50 of 0.0012 mg/cm2 (95% CI: 0.0003–0.0029) by Robert et al. on rabbits [10], 0.00056 mg/cm2 (95% CI: 0.00041–0.00072) by Coleman et al. [21] and 0.00013 mg/cm2 (95% CI: 0.000003–0.00059) by Klun and Debboun on humans [20].
Regarding relationship between dose and biting/probing on repellent-treated rabbits against the laboratory strain of An. stephenis, the dose–response regression line showed significant heterogeneity for DEET. This indicates a wider range of tolerance of mosquitoes exposed to DEET.
Cosmetic acceptability is the most important criterion in the widescale use of topical insect repellents. DEET has gained wide acceptance and is used in many countries throughout the world. It is the most effective and best studied insect repellent currently on the market. This substance has a remarkable safety profile after 40 years of worldwide use, but toxic reactions can occur, usually when the product is misused [22]. However, a comparison of the mosquito repellant efficacy of methyl neodecanamide (MNDA) to that of DEET on 3 species including An. stephensi indicated that topical application of 1% MNDA provided significantly better protection and a broader spectrum of repellency than 1% DEET [23].
The effectiveness of permethrin as a clothing impregnation or pressurized spray for personal protection against mosquitoes has been demonstrated [24,25]. This component has low toxicity in mammals, is poorly absorbed by the skin and is rapidly inactivated by ester hydrolysis [22].
Synthetic repellents are routinely used for prevention of arthropod bites in the Islamic Republic of Iran. Our results indicated that DEET is a more effective repellent against both laboratory and field strains of An. stephensi than neem or permethrin. DEET-based repellents applied on the skin in combination with permethrin-treated clothing could provide more protection against mosquito bites [26]. Ongoing field trails on human volunteers will provide valuable information and insights into the role of particular repellents in preventing mosquito biting, especially for travellers coming into malarious areas.
Natural plant extracts have been used for centuries by local people to prevent arthropod bites. Our results showed that the plant-based repellent was generally less effective than the synthetic repellents. Although it was the least effective agent, extracts of locally produced neem oil offer a promising repellent against biting. Our previous study on larvicidal activity of neem against different species of mosquitoes showed good results in the Islamic Republic of Iran [27]. The main implications of this study are that in malarious area where An. stephensi plays an important role in malaria transmission, the local plant can be used in combination with other synthetic chemicals for reducing of malaria vector density and human–mosquito contact, resulting in reduction of the vectorial capacity of the mosquito.
Acknowledgements
We are grateful to Mr A. Pakari, Mr H. Shabkhiz, Mr H. Javdan and other personnel of Bandar Abbas Research Centre for their kind assistance in the current study. Our greatest thanks are due to Dr Hajiakhondi from the Faculty of Pharmacology for providing the neem extract. This project has received financial support from the Institute of Public Health Research, School of Public Health as an academic pivot, Tehran University of Medical Sciences, Project No. 241774.
References
- Service MW. Mosquitoes (Culicidae). In: Lane RP, Crosskey RW, eds. Medical insects and arachnids. London, Chapman and Hall, 1993:723.
- Gupta RK, Rutledge LC. Role of repellents in vector control and disease prevention. American journal of tropical medicine and hygiene, 1994, 50:82–6.
- Gupta RK, Rutledge LC. Laboratory evaluation of controlled-release repellent formulations on human volunteers under three climatic regimens. Journal of the American Mosquito Control Association, 1989, 5:52–5.
- Schmutterer H. Properties and potential of natural pesticides from neem tree, Azadirachta indica. Annual review of entomology, 1990, 35:271–97.
- Ladd TL, Jacobson M, Buriff CR. Japanese beetles: extracts from neem tree seeds as feeding deterrents. Journal of economic entomology, 1978, 77:810–3.
- Sharma VP, Dhiman RC. Neem oil as a sand fly (Diptera: Psychodidae) repellent. Journal of the American Mosquito Control Association, 1993, 9(3):364–6.
- Sharma VP, Ansari MA, Razdan RK. Mosquito repellent action of neem (Azadirachta indica) oil. Journal of the American Mosquito Control Association, 1993, 9:359–60.
- Yap HH. Effective of soap formulations containing deet and permethrin as personal protection against outdoor mosquitoes in Malaysia. Journal of the American Mosquito Control Association, 1986, 2(1):63–7.
- Gupta RK et al. Effects of weathering on fabrics treated with permethrin for protection against mosquitoes. Journal of the American Mosquito Control Association, 1989, 5(2):176–9.
- Robert LL et al. Comparative sensitivity of four Anopheles (Diptera: Culicidae) to five repellents. Journal of medical entomology, 1991, 28(30):417–20.
- Kroeger A et al. The contribution of repellent soap to malaria control. American journal of tropical medicine and hygiene, 1997, 56(5):580–4.
- Cockroft A, Cosgrove JB, Wood RJ. Comparative repellency of commercial formulations of deet, permethrin and citronellal against the mosquito Aedes aegypti, using a collagen membrane technique compared with human arm tests. Medical and veterinary entomology, 1998, 12(3):289–94.
- Klun JA et al. Synthesis and repellent efficacy of a new chiral piperidine analog: comparison with Deet and Bayrepel activity in human-volunteer laboratory assays against Aedes aegypti and Anopheles stephensi. Journal of medical entomology, 2003, 40(3):293–9.
- Rutledge LC et al. Comparative sensitivity of mosquito species and strains to the repellent diethyl toluamide. Journal of medical entomology, 1978, 14:536–41.
- Reifenrath WG, Akers WA. Field testing of repellents against anopheline mosquitoes. Mosquito news, 1981, 41:276–80.
- Rutledge LC et al. Comparative sensitivity of representative mosquitoes (Diptera: Culicidae) to repellents. Journal of medical entomology, 1983, 20:506–10.
- Thavara U et al. Laboratory and field evaluations of the insect repellent 3535 (ethyl butylacetylaminopropionate) and deet against mosquito vectors in Thailand. Journal of the American Mosquito Control Association, 2001, 17(3):190–5.
- Manouchehri AV, Djanbakhsh E, Rouhani F. Studies on the resistance of Anopheles stephensi to malathion in Bandar Abbas, Iran. Mosquito news, 1976, 36:320–2.
- Vector resistance to pesticides. Fifteenth report of the WHO Expert Committee on Vector Biology and Control. Geneva, World Health Organization, 1992 (WHO Technical Report Series No. 818).
- Klun JA, Debboun M. A new module for quantitative evaluation of repellent efficacy using human subjects. Journal of medical entomology, 2000, 37:177–81.
- Coleman RE et al. Laboratory evaluation of repellents against four anopheline mosquitoes (Diptera: Culicidae) and two phlebotomine sandflies (Diptera: Psychodidae). Journal of medical entomology, 1993, 30:499–502.
- Mark S, Fradin MD. Mosquitoes and mosquito repellents: a clinician’s guide. Annals of internal medicine, 1998, 128:931–40.
- Polefka TG, Liang LJ, Ananthakrishnan TN. Comparison of the mosquito–repelling efficacy of methyl neodecanamide (MNDA) to that of Deet. Journal of cosmetic science, 2003, 54(3):283–8.
- Lindsay LS, McAndless JM. Permethrin-treated jackets versus repellent-treated jackets and hoods for personal protection against blackflies and mosquitoes. Mosquito news, 1978, 38:350–6.
- Schreck CE, Haile DG, Kline DL. The effectiveness of permethrin and deet, alone or in combination, for protection against Aedes taeniorhynchus. American journal of tropical medicine and hygiene, 1984, 33:725–30.
- Deparis X et al. Efficacy of permethrin-treated uniforms in combination with DEET topical repellent for protection of French military troops in Cote d’Ivoire. Journal of medical entomology, 2004, 41(5):914–21.
- Vatandoost H, Vaziri VM. Larvicidal activity of a neem tree extract (Neemarin) against mosquito larvae in the Islamic Republic of Iran. Eastern Mediterranean health journal, 2004, 10(4/5):573–81.


