A. Aminorroaya,1 M. Momenzadeh,1 S. Hovsepian,1 S. Haghighi1 and M. Amini1
الأضداد الذاتية في المصابات وغير المصابات باضطرابات الدرقية في منطقة يعوَّض فيها اليود
أشرف أمين الرعايا، مهين مؤمن زاده، سيلوا هوسبيان، ساسان حقيقي، مسعود أميني
الخلاصـة: كان الهدف من هذه الدراسة مقارنة معدل انتشار الأضداد الذاتية الإيجابية في المصابات وغير المصابات باضطرابـات الدرقيـة، في إحـدى المناطق التي يعوَّض فيهـا اليود في جمهوريـة إيران الإسلاميـة. وقد شملت الدراسـة 930 مريضة (منهن 286 مصابة بقصور الدرقية و140 مصابة بفرط نشاط الدرقية و272 مصابة بدُراق goiter بسيط)، إلى جانب 232 من الأصحاء. وأجريت قياسات للثيروكسين T4 وثلاثي يودوثيرونين T3 والهرمون المنبه للدرقية وكذلك لأضداد الدرقية، واكتشفت إيجابية الأضداد الذاتية لدى 75.5% من المصابات بقصور الدرقية ولدى 73.6% من المصابات بفرط نشاط الدرقية، ولدى 48.9% من المصابات بدُراق بسيط ولدى 35.8% من مجموعة الشاهدات المعافيات (0.001<). p="">
ABSTRACT: To compare the prevalence of positive autoantibodies in patients with thyroid disorders and healthy subjects in an iodine-replete area of the Islamic Republic of Iran, we studied 930 women in a clinic-based study: 698 patients (286 hypothyroid, 140 hyperthyroid, 272 with simple goitre) and 232 healthy women. Serum thyroxine (T4), triiodothyronine (T3), thyroid stimulating hormone, and anti-thyroid antibodies were measured. Positive autoantibodies were detected in 75.5% of patients with hypothyroidism, 73.6% of those with hyperthyroidism, 48.9% of those with simple goitre and 35.8% of the control group (P < 0.001). Autoimmunity may have a role in the genesis of common thyroid disorders.
Autoanticorps thyroïdiens chez les femmes avec et sans troubles thyroïdiens dans une région riche en iode
RÉSUMÉ: Afin de comparer la prévalence des autoanticorps positifs chez les patients atteints de troubles thyroïdiens et chez des sujets en bonne santé dans une région riche en iode de la République islamique d’Iran, nous avons effectué une étude clinique sur 930 femmes : 698 patientes (286 hypothyroïdiennes, 140 hyperthyroïdiennes et 272 présentant un goitre simple) et 232 femmes en bonne santé. La thyroxine (T4), la triiodothyronine (T3), la thyréostimuline, ou TSH (pour thyroid stimulating hormone) sériques et les anticorps antithyroïdiens ont été mesurés. Des autoanticorps positifs ont été détectés chez 75,5 % des patientes atteintes d’hypothyroïdie, 73,6 % de celles atteintes d’hyperthyroïdie, 48,9 % de celles présentant un goitre simple et 35,8 % dans le groupe témoin (p < 0,001). L’auto-immunité peut avoir un rôle dans l’apparition des troubles thyroïdiens courants.
1Isfahan Endocrine and Metabolism Research Centre, Isfahan University of Medical Sciences, Isfahan, Islamic Republic of Iran (Correspondence to A. Aminorroaya:
Received: 16/11/05; accepted: 23/01/06
EMHJ, 2008, 14(2): 325-332
Introduction
Iodine deficiency disorders were prevalent in the Islamic Republic of Iran prior to 1989 when the national salt iodization programme was initiated [1]. A law for the mandatory production of iodized salt for households was passed in 1994. Since then, the country has achieved a sustainable control programme for iodine deficiency [1,2].
Isfahan is a centrally located city in the Islamic Republic of Iran with a population of about 1 000 000. In a study conducted on 3000 Isfahan students after 8 years of iodine repletion, 94% had sufficient iodine consumption [2]. Despite this, however, the prevalence of goitre was still high (63%) [2]. So the question of iodine-induced autoimmune thyroid disease was raised [2,3]. A randomized, double-blind, placebo-controlled trial reported the transient occurrence of high levels of thyroid antibodies in 6 of 31 patients with endemic goitre treated daily for 6 months with a supraphysiologic dose of 500 μg/day iodine. Therefore, it was concluded that iodine may induce thyroid autoimmunity in patients with endemic goitre [3].
The clinical implications of thyroid autoimmunity following correction of iodine deficiency are, however, still unclear [4]. In a survey done in Shahriar, Islamic Republic of Iran, positive antithyroid antibodies were observed in 3.1% of the population before iodine supplementation [5]. In a clinic-based study in Shiraz in 1981, antithyroid antibodies were found in 2% of healthy volunteers, 3% of patients with simple goitre, 41% of those with hyperthyroidism and 67% of those with hypothyroidism [6].
As high titres of antithyroid antibodies are strongly suggestive of thyroid autoimmunity [7], we investigated the frequency of thyroid autoantibodies 12–14 years after iodine repletion in Isfahan by measuring these antibodies in patients with thyroid disorders and in a healthy control group.
Methods
Participants
Women were chosen for this study as it is known they have a higher prevalence of thyroid autoimmune markers and goitre compared with men [7]. Isfahan Endocrine and Metabolism Research Centre is a referral centre for endocrine disorders. All 990 women in Isfahan who met the inclusion criteria and who were referred to the outpatient endocrine clinic during the period April 2001–March 2003 were invited to participate in the study; 930 agreed and gave voluntary informed consent. Those who were pregnant or on medications which would affect thyroid function tests (e.g. oral contraceptive pills, levothyroxine or thionamides) were excluded.
Participants were classified into 4 groups, hypothyroidism, hyperthyroidism, simple goitre, and a healthy control group (no goitre and normal thyroid function). The women in the control group were those with certain nonspecific symptoms who had been referred with suspected thyroid disorder, but after history-taking and physical examination, were diagnosed as completely healthy and their thyroid function tests were normal.
Initial clinical evaluation included taking demographic data, noting symptoms of hypo- and hyperthyroidism, physical examination for signs of thyroid dysfunction and presence or absence of goitre. A thyroid gland whose lateral lobes had a volume greater than the terminal phalanges of the thumbs of the person examined was considered goitrous [4].
The protocol was approved by the Institutional Review Board and Medical Ethics Committee of Isfahan University of Medical Sciences.
Laboratory measurements
Commercially-available kits were used to measure serum concentrations of thyroxine (T4) and triiodothyronine (T3) by radioimmunoassay using a gamma counter (Berthold Technologies GmbH, Germany), and thyroid-stimulating hormone (TSH) by immunoradiometric assay (Kavoshyar, Tehran). The normal (reference) ranges were: T4: 5.50–11.03 µg/dL; T3: 80–200 ng/dL; TSH: 0.36–3.68 mIU/L. Those with TSH values > 3.68 mIU/L were diagnosed as having hypothyroidism. Those with T4 values > 11.03 µg/dL and/or T3 > 200 ng/dL and TSH < 0.36 mIU/L were diagnosed as having hyperthyroidism. Simple goitre was defined as presence of goitre with normal thyroid function (T4, T3 and TSH within normal range).
Serum antithyroid peroxidase (anti-TPO/TPOAb) and antithyroglobulin (anti-Tg/TgAb) were measured by enzyme-linked immunosorbent assay standardized against the WHO reference preparation 66/387 for anti-TPO and 65/93 for anti-Tg antibodies (DRG Instruments GmbH, Germany). The intra-assay variation was 5.3% for TPOAb and 3.3% for TgAb. The inter-assay variation for was 2.0% TPOAb and 4.0% for TgAb.
Serum TPOAb 75IU/mL as elevated (positive). Serum TgAb 150 IU/mL as elevated (positive).
Statistical analysis
Data with normal distribution were shown as mean and standard deviation (SD), unless stated otherwise. We compared variables using analysis of variance, chi-squared and t-tests as appropriate using SPSS, version 12, and Epi-Info, version 6.04. P < 0.05 was considered statistically significant.
Results of variables not distributed normally are shown as box plots with 25th, 50th and 75th percentiles.
Results
Mean age in hypothyroid, hyperthyroid, simple goitre and control groups was 27.0 (SD 12.1), 30.1 (SD 13.5), 28.2 (SD 13.9) and 27.3 (SD 13.1) years, respectively. There was no difference in age between groups.
Mean levels for serum T4, T3 and TSH are shown in Table 1. As expected, mean T4 and T3 levels were statistically significantly lower in hypothyroid women and statistically significantly higher in the hyperthyroid group than in the control group (P < 0.05). Mean TSH level was higher in hypothyroid patients than in those who were euthyroid, and was suppressed in those with hyperthyroidism.
Medians for TPOAb and TgAb were higher in the hypo- and hyperthyroidism and simple goitre groups than in the control group. The distribution of autoantibody concentration is shown as box plots (Figures 1 and 2). Median (range) for TPOAb in these groups was 200 (< 1–8361), 200 (5–5200), 30 (< 1–2975) and 20 (< 1–2336) IU/mL, respectively and for TgAb was 197 (1–9317), 165 (4–5500), 67 (< 1–4219) and 27 (< 1–4219) IU/ mL, respectively.
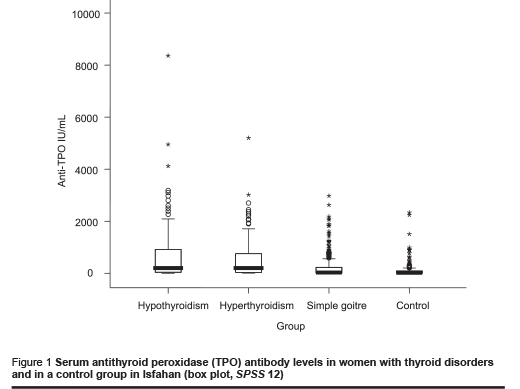
Frequency of positive antithyroid antibodies was statistically significantly higher in the hypo- and hyperthyroidism groups than in the control group (Table 2). This was also higher in the simple goitre group, but not statistically significantly. Frequency of positive TPOAb and/or TgAb was similar in the hypo- and hyperthyroidism groups. Frequency of positive autoantibodies did not increase with age in patients with thyroid disorders or in the control group.
Negative thyroid antibody levels were lower in the hypothyroidism (P < 0.001), hyperthyroidism (P < 0.001) and simple goitre (P < 0.05) groups than in the control group (Table 2). The difference was statistically significant except for TPOAb in the simple goitre group.
Only-positive TPOAb was detected in 22.0% of those with hypothyroidism, 20.7% of those with hyperthyroidism, 16.5% of those with simple goitre and 14.7% of the control group. For only-positive TgAb, corresponding values were 9.4%, 11.4%, 11.8% and 9.1%, respectively. Only-positive TPOAb was more frequent than only-positive TgAb in the hypo- (P < 0.001) and hyperthyroidism (P < 0.05) groups.
Of those women with positive TPOAb, 66.7% in the hypothyroidism group, 65.9% in the hyperthyroidism group, 55.4% in the simple goitre group and 42.4% of the control group also had positive TgAb; 82.4%, 77.8%, 63.6% and 54.3% of those with positive TgAb in these groups also had positive TPOAb. The difference was not statistically significant except in the hypothyroid group (P < 0.001).
Discussion
In this study, thyroid autoantibodies were measured in women referred to an endocrine clinic in Isfahan, an iodine-replete area. Serum concentrations of both TPOAb and TgAb were much higher in all 3 groups with thyroid disorders in comparison with the healthy control group.
Prevalence of positive antithyroid antibodies (TPOAb and/or TgAb) was 75.5%, 73.6%, 48.9% in patients with hypothyroidism, hyperthyroidism and simple goitre. It was much higher than in the control group (35.8%), although this was also high. In a clinic-based study in Shiraz, Islamic Republic of Iran, during the iodine deficient period (1981), corresponding values were 67%, 41%, 3% and 2% (control group) [6]. In our study in Isfahan, after iodine supplementation, the prevalence of positive antithyroid antibodies was much higher than in Shiraz in all groups. The concentration of these antibodies had not been measured in Isfahan before iodine supplementation so we could not compare our data with previous findings for the same population. This is one of the limitations of this study.
In a Greek study, carried out 7 years after iodine repletion, a 3-fold increase in the prevalence of autoimmune thyroiditis was observed among schoolchildren evidenced by TPOAb and/or TgAb levels [8]. In Iran, frequency of positive thyroid autoantibodies was 2% in Shiraz in a disease-free population before iodine supplementation [6]. In our sample in Isfahan, after elimination of iodine deficiency, it was 35.6% in healthy subjects, 18 times higher after iodine repletion.
In Zimbabwe, an iodine-replete country, 39% of hyperthyroid patients had positive TPOAb or TgAb. None of these antibodies was positive in patients with simple goitre [9]. In Isfahan, it was 75.5% and 48.9% in hypothyroid and simple goitre patients. It seems that iodine prophylaxis-induced autoimmune thyroid disorders are more prevalent in Isfahan than Zimbabwe, even in goitre without thyroid dysfunction. These simple goitres may progress to hypo- and hyperthyroidism in future.
In this study from Isfahan, TPOAb was positive in 66.3%, 62.1% and 26.8% of women with hypo- and hyperthyroidism, simple goitre and control groups, respectively. In a recent study in Tehran on women with postpartum thyroiditis and a control group, positive anti-TPO was observed in 61.5% and 19.0% respectively [10]. Prevalence of positive autoantibodies was similar in women with thyroid dysfunction in Tehran and Isfahan, but was higher in the Isfahan control group (Table 2).
In Pakistan, positive TPOAb was observed in 78.9% of patients with Graves’ disease and in 13.3% of the control group; positive TgAb was observed in 57.9% in patients and 6.7% of the control group [11]. Prevalence of positive antibodies in those with thyroid disorders was similar to the findings in our study, but lower in the control group. While the prevalence of thyroid autoantibodies is generally higher in women, and all participants in our study were female [12–14], higher prevalence of antibodies in healthy Isfahani subjects compared with Pakistani people cannot be explained by patient selection as higher prevalence of positive antibodies has been observed in disease-free women in Tehran, too [10]. In comparison to reports from Zimbabwe, Pakistan and Tehran, prevalence of positive antibodies in the control group was higher in Isfahan [9–11]. It appears that Isfahani people are more susceptible to thyroid autoimmunity.
In Sri Lankan schoolgirls, the prevalence of TgAb has been markedly raised since iodine supplementation, ranging between 14.3% (11-year-old girls) and 69.7% (16-year-old girls), but the prevalence of TPOAb was ≤ 10% in all age groups [14]. In our study, the prevalence of positive antibodies did not increase with age in any group. In Thailand, prevalence of anti-thyroid antibodies was higher in an elderly compared to a younger age group (14.69% versus 5.02%) [12]. Prevalence of thyroid antibodies in the population of the United States of America, National Health and Nutrition Examination Survey (NHANES III) study, increased with age [13]. These 3 studies were population-based but ours was clinic-based. This might explain why we fond no correlation between age and positive antithyroid autoantibodies.
In the NHANES III study and also in a study from Bangladesh, TgAb alone in the absence of TPOAb was not significantly associated with thyroid disease [13,15]. In Budapest, 57.9% of hyperthyroid patients who had positive anti-TPOAb were negative for anti-TgAb, whereas 9.4% of those with positive anti-TgAb were anti-TPOAb negative [16]. Corresponding values were 22.0% and 9.4% respectively in our study.
In recent study, only-positive TPOAb was more prevalent than only-positive TgAb in hypo- and hyperthyroidism, but the difference was not as great as in other studies [13,15,16]. However, the higher prevalence of TPOAb compared to TgAb and both combined, similar to the above studies, makes measurement of anti-Tg superfluous. Prevalence of negative anti-thyroid antibodies was much higher in the control group, and serum titres of these anti-bodies were significantly higher in those with thyroid disorders than in the control group in Isfahan, Islamic Republic of Iran.
Although the findings of the present study may suggest that iodine-induced autoimmune thyroid disease is increasing in the Islamic Republic of Iran since iodine repletion, a cohort population-based study is recommended to investigate this possibility. It appears that TPOAb measurement would be enough to survey thyroid autoimmunity.
Acknowledgement
We are grateful to M. Majid Abyar for computer technical assistance.
References
- Azizi F et al. Sustainable control of iodine deficiency in Iran: beneficial results of the implementation of the mandatory law on salt iodization. Journal of endocrinological investigation, 2002, 25(5):409–13.
- Aminorroaya A et al. Effects of iodized salt consumption on goiter prevalence in Isfahan: the possible role of goitrogens. Endocrine practice, 2001, 7(2):95–8.
- Kahaly GJ et al. Iodide induces thyroid autoimmunity in patients with endemic goitre: a randomised, double-blind, placebo-controlled trial. European journal of endocrinology, 1998, 139(9):290–7.
- Delonge FM. Iodine deficiency. In: Braverman LE, Utiger RD, eds. Werner & Ingbar’s The thyroid, 8th ed. Philadelphia, Lippincott Williams & Wilkins, 2000:295–316.
- Azizi F, Navai L, Fattahi F. Goiter prevalence, urinary iodine excretion, thyroid function and anti-thyroid function and anti-thyroid antibodies after 12 years of salt iodization in Shahriar, Iran. International journal for vitamin and nutrition research, 2002, 72:291–5.
- Khaleeli AA. Prevalence of thyroid antibodies in Shiraz, Iran, an area with iodine deficiency. Postgraduate medical journal, 1981, 57(663):23–7.
- Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. New England journal of medicine, 1996, 335:99–107.
- Zois C et al. High prevalence of autoimmune thyroiditis in schoolchildren after elimination of iodine deficiency in northwestern Greece. Thyroid, 2003, 13:485–9.
- Chinyanga EA, Benni A, Siziya S. Thyroid status and the levels of thyroid auto-antibodies in the sera of hyperthyroid and goitrous subjects. Central African journal of medicine, 2000, 46(9):251–5.
- Shahbazian HB, Sarvghadi F, Azizi F. Prevalence and characteristics of postpartum thyroid dysfunction in Tehran. European journal of endocrinology, 2001, 145:397–401.
- Tayyab M, Bhatti KU, Ditta A. Prevalence of thyroid microsomal and thyroglobulin autoantibodies in goitrous lesions. Journal of Ayub Medical College, Abbottabad, 2001,13(3):16–8.
- Hanvivatvong O et al. Prevalence of autoantibodies in Thai elderly. Journal of the Medical Association of Thailand, 2003, 86(Suppl. 2):S242–9.
- Hollowell JG et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). Journal of clinical endocrinology & metabolism, 2002, 87(2):486–8.
- Premawardhana LD et al. Increased prevalence of thyroglobulin antibodies in Sri Lankan schoolgirls—is iodine the cause? European journal of endocrinology, 2000, 143(2):185–8.
- Hasanat MA et al. Status of antithyroid antibodies in Bangladesh. Postgraduate medical journal, 2000, 76(896):345–9.
- Foldes I, Levay A. [Antibodies against thyroid gland peroxidase and thyroglobulin in various thyroid diseases]. Orvosi hetilap, 1994, 135(29):1579–84 [in Hungarian].


