R.R. Hafidh1 and A.S. Abdulamir2
1Department of Microbiology, College of Medicine, University of Baghdad, Baghdad, Iraq (Correspondence to R.R. Hafidh:
2Department of Microbiology, College of Medicine, Al-Nahrain University, Baghdad, Iraq.
Received: 02/10/05; accepted: 15/12/05
EMHJ, 2008, 14(1): 231-233
Introduction
White superficial onychomycosis is less common than distal subungual onychomycosis and occurs when certain fungi invade the superficial layers of the nail plate directly. Later, the infection may move through the nail plate to infect the cornified layer of the nail bed and hyponychium. It can be recognized by the presence of well-delineated opaque “white islands” on the external nail plate, which coalesce and spread as the disease progresses. At this point, the nail becomes rough, soft and crumbly. Inflammation is usually minimal in patients with white superficial onychomycosis, because viable tissue is not involved. It occurs primarily in the toenails.
The most common etiologic agent in white superficial onychomycosis is Trichophyton mentagrophytes. Also, several non-dermatophyte moulds, including Aspergillus terreus, Acremonium roseogrisum (later confirmed to be Acremonium potronii) and Fusarium oxysporum, have been implicated [1].
We aimed to detect the involvement of some non-dermatophytic filamentous fungi in onychomycosis and reports on a case of onychomycosis due to Cladosporium spp.
Methods
Thirty-six patients (36) with onychomycosis were selected from the outpatient dermatological clinic of Al-Kadymia Teaching Hospital in Baghdad, Iraq.
Sample specimens were collected after cleansing the nail area with alcohol. Different approaches, depending on the presumptive diagnosis, were necessary to obtain optimal specimens.
The common distal-subungual type of onychomycosis was sampled after light alcohol disinfection by scraping the debris from beneath the distal end of the nail with a scalpel and collecting scrapings from near the nail bed, where viable inoculum is most likely to be encountered. Close clipping of the whole nail end was an alternative to this procedure.
Superficial white onychomycosis was sampled by scraping material from the white spots on the surface of the nail. Discarding the uppermost layer of material is recommended in order to reduce the presence of contaminant inoculum [2]. A potassium hydroxide solution containing dimethyl sulfoxide was used to clear thick pieces of nail tissue [3].
Nail scrapings were cultured on Sabouraud dextrose agar slant with 0.05% cycloheximide to inhibit contaminating fungi and allow growth of dermatophytes. Sabouraud dextrose agar without cycloheximide was also used to enable the growth of nondermatophyte fungi that are commonly considered saprophytes or contaminants but can act as opportunistic pathogens. These were incubated at 30 °C and examined every 2–3 days. Negative cultures were discarded after 4 weeks.
Many typical isolates of common dermatophytes can be identified directly from primary isolation media, particularly Sabouraud’s dextrose agar. Identification characteristics include colony pigmentation, texture and growth rate and distinctive morphological structures, such as microconidia, macroconidia, spirals, pectinate branches, pedicels and nodular organs.
A standard germ tube test was used for identification of Candida albicans. Biochemical procedures were used to further identify Candida species. Moulds forming colonies on other separate plates were fully identified.
Results
The culture results of the 36 patients with onychomycosis are shown in Table 1.
A 52-year-old woman with white superficial toenail onychomycosis was diagnosed to have Cladosporium spp. as a causative agent for her onychomycosis infection. Potassium hydroxide preparations revealed the presence of hyaline septate hyphae (Figure 1). Cladosporium spp. grew rapidly; colonies appeared as velvety, heaped and folded. Hyphae were septate and brown. Conidiophores were long and branched and gave rise to chains of darkly pigmented budding conidia. Conidia are usually single-celled and exhibit prominent attachment scars that may resemble “shield” cells. It was difficult to observe the chains of conidia on wet mount because the conidia dislodge easily.
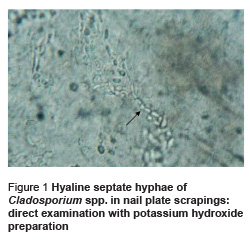
Discussion
The last 3 decades have seen unequivocal documentation of the role of non-dermatophytes as causal agents in onychomycosis. The most common yeast that is involved is Candida albicans. The non-dermatophytic filamentous fungi, agents implicated in onychomycosis, include Scopulariopsis spp. (particularly Scopulariopsis brevicaulis) and Scytalidium (the 2 most common genera being: Scytalidium hyalinum, Scytalidium demidiatum), which are both thought to digest keratin in vivo, as well as members of the genera Alternaria, Aspergillus, Acremonium and Fusarium. Many of these non-dermatophyte fungi invade the nail unit directly and cause white subungual onychomycosis [4,5].
Known risk factors for the increased incidence of non-dermatophytic filamentous fungi onychomycosis include foot dampness and abrasion combined with likely exposure to high fungal inoculum [6].
Piraccini and Tosti found that white superficial onychomycosis may have different clinical and epidemiological features [7]. “Classic” white superficial onychomycosis, characterized by superficial nail plate involvement, is usually due to Trichophyton mentagrophytes (var interdigitale), although Acremonium strictum or Onychocola canadiensis can sometimes be responsible. A deep and diffuse white superficial onychomycosis, characterized by massive penetration of the nail plate by fungi, can be seen in nail infections by moulds such as Fusarium species and Aspergillus species, or in nail infections by Trichophyton rubrum in healthy children and in patients infected with human immunodeficiency virus.
Part of the difficulty in evaluating the role of non-dermatophytic fungi cultured from the nail arises because the same fungi that can be laboratory contaminants are also occasionally found to be pathogens. All dermatophytes should be considered, as pathogens all other isolated organisms are probably laboratory contaminants unless potassium hydroxide or microscopy indicates the presence of septate hyaline hyphae associated with non-dermatophyte moulds or if the same organism is repeatedly isolated [5].
We found only 1 case of non-dermatophytic onychomycosis by filamentous fungi with the genus Cladosporium out of 36 cases of onychomycosis. Lestringant et al. isolated Cladosporium spp. from only 1 patient out of 45 with toe-web disease in Tawam Hospital, United Arab Emirates [8]. Their results confirm ours of the chances of isolating the filamentous fungus Cladosporium spp. from onychomycosis, toe-web disease, or both, considering Cladosporium spp. as a sole source for this type of fungal infection.
References
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clinical microbiology reviews, 1998, 11:415–29.
- Weitzman I, Summerbell RC. The dermatophytes. Clinical microbiology reviews, 1995, 82:240–59.
- McGinnis MR. Laboratory handbook of medical mycology. New York, Academic Press, 1980.
- Summerbell RC, Kane J, Krajden S. Onychomycosis, tinea pedis, and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses, 1989, 32:609–19.
- Summerbell RC. Epidemiology and ecology of onychomycosis. Dermatology, 1997, 194:32–6.
- Auger P et al. Epidemiology of tinea pedis in marathon runners: prevalence of occult athlete’s foot. Mycoses, 1993, 36:35–41.
- Piraccini BM, Tosti A. White superficial onychomycosis: epidemiological, clinical, and pathological study of 79 patients. Archives of dermatology, 2004, 140(6):696–701.
- Lestringant GG et al. Etiology of toe-web disease in Al-Ain, United Arab Emirates: bacteriological and mycological studies. Eastern Mediterranean health journal, 2001, 7(1/2):38–45.


