F. Ghaffarifar,1 O. Jorjani,1 M. Mirshams,2 M.H. Miranbaygi3 and Z.K. Hosseini4
ABSTRACT We assessed the effectiveness of photodynamic therapy in the treatment of cutaneous leishmaniasis in 5 patients. Delta-aminolevulinic acid in a water-in-oil emulsion was applied to the lesions and irradiation was performed. The treatment was repeated once a week for a month. Each time, direct smears of the lesions were prepared and cultured in NNN media. In direct staining, smears showed no amastigotes after 1 or 2 sessions. Healing and cosmetic outcome after photodynamic therapy was excellent. Only mild local inflammatory reaction was noted with no scarring and 4 months after the last treatment session, there were no clinical signs of recurrence.
La thérapie photodynamique comme nouveau traitement de la leishmaniose cutanée
RÉSUMÉ Nous avons évalué l’efficacité de la thérapie photodynamique dans le traitement de la leishmaniose cutanée chez 5 patients. L’acide delta-aminolévulinicque (ALA) dans une émulsion eau/huile a été appliqué sur les lésions et une irradiation a été réalisée. Le traitement a été répété une fois par semaine pendant un mois. À chaque fois, des frottis directs des lésions ont été préparés et mis en culture dans un milieu NNN. En coloration directe, les frottis n’ont montré aucun amastigote après 1 ou 2 séance(s). La cicatrisation et le résultat sur le plan esthétique après la thérapie photodynamique étaient excellents. Seule une légère réaction inflammatoire locale a été notée sans formation de cicatrices et 4 mois après la dernière séance de traitement, il n’y avait aucun signe clinique de récidive.
1Department of Parasitology, Medical School, Tarbiat Modarres University, Tehran, Islamic Republic of Iran (Correspondence to F. Ghaffarifar:
2Department of Dermatology, Tehran Medical University, Razi Hospital, Tehran, Islamic Republic of Iran.
3Department of Medical Engineering, Technical and Engineering School, Tarbiat Modarres University, Tehran, Islamic Republic of Iran.
4Razi Hospital, Tehran, Islamic Republic of Iran.
Received: 28/02/05; accepted: 10/05/05
EMHJ, 2006, 12(6): 902-908
Introduction
Cutaneous leishmaniasis is an important health problem caused by the flagellated protozoa, Leishmania major and L. tropica. Leishmania is transmitted by female sandflies, Phlebotomus species [1]. Cutaneous leishmaniasis occurs 1–12 weeks after exposure with a small inconspicuous papule on the exposed site which enlarges and finally ulcerates [1–3].
As there is no vaccine, drug treatment is the only way to tackle leishmaniasis. The drugs of choices are sodium stibogluconate and meglumine antimonite (both pentavalent antimony derivatives). Pentavalent antimony compounds have been in use for more than half a century, and they have shown response rates between 72% and 100% after intralesion application [1,4,5]. Their mechanism of action is not completely known. Development of resistance is of increasing concern; they require weeks of intravenous administration and are frequently associated with malaise, anorexia, myalgia and arthralgia, electrocardiographic abnormalities, elevated aminotransferase levels, and chemical pancreatitis [1].
Amphotericin B is recommended as second-line treatment. The most dangerous side-effect of amphotericin B is kidney damage [4–6]. Other alternative treatments exist, including cryotherapy, excision, curettage and electrodesiccation, but they bear a higher risk of recurrence and are cosmetically unsatisfactory. Unfortunately, no ideal therapy for the cutaneous leishmaniasis has yet been identified [1,3,5,7–9].
Photodynamic therapy is a developing technique which uses light to induce reactions in the body which are of benefit to patients. Photodynamic therapy uses a drug (photosensitizing agent) and a particular type of light to destroy diseased
tissue/cells whilst sparing normal tissue. When photosensitizers are exposed to a specific wavelength of light, they produce a form of oxygen that kills nearby cells [10–12]. Each photosensitizer is activated by light of a specific wavelength [12,13]. This wavelength determines how far the light can travel into the body [12–14]. Thus, specific photosensitizers and wavelengths of light are used to treat different areas of the body with photodynamic therapy.
Light technology and its use in dermatology have grown rapidly in the last decade. There have been many developments in the use of light for the treatment of a wide variety of skin conditions from non-melanoma skin cancers [15–18] to facial resurfacing for crows’ feet and photo-damaged skin [19–21].
The aim of this study was to assess photodynamic therapy in the treatment cutaneous leishmaniasis in terms of clearance of lesions, cosmetic outcome and recurrence.
Methods
This study was carried out at Razi Hospital from January 2004 until June 2004. Five (5) patients with confirmed cutaneous leishmaniasis (Leishmania sore in arm, forearm or leg) were selected: 3 patients had 1 lesion and 2 had 2 lesions. Keratotic plaques had developed 1 to 3 months before presentation to our department and no treatment had been used.
A smear was prepared from the lesion of the patients and Geimsa-stained. Another specimen from the lesions was cultured in NNN media. Cultivation of promastigote in NNN media was for confirmation of eradication of amastigotes. The culture was investigated daily, and when cutaneous leishmaniasis was confirmed, the patients infected with L. major were selected to receive photodynamic therapy [22].
For each patient, 10% delta-aminolevulinic acid (ALA) in a water-in-oil emulsion (0.25 g crystal of ALA, 0.25 mL distilled water, 2 g Unguentum as carrier material) was applied locally to the lesions under occlusion for 4 hours. The thickness of the emulsion on the sore was 1–2 mm and it was spread over a radius of 5 mm. The ALA selectively accumulated on the leishmaniasis lesions as demonstrated by fluorescence of ALA-induced porphyrins after exposure to Wood light. Irradiation was performed using broadband red light (570–670 nm), delivering 100 J/cm2 per treatment session at a light intensity of 150 mW/cm2 (approximately 21 minutes). Distance between the light source and skin surface was 20 mm.
The treatment was repeated once a week for a month. Each time, direct smears of the lesions were prepared and cultured in NNN media [23].
The lesions were assessed clinically as follows:
- The size was measured by vernier caliper (mean of 2 diameter measurements).
- Evaluation of inflammation was judged by erythema and swelling.
- Evaluation of epithelialization was based on crust and exudates.
Results
In the lesions of 3 patients after 1 treatment session and in the lesions of 2 patients after 2 sessions, the amastigotes were no longer detectable. There was no recurrence of amastigotes during the observation period (4 months). So photodynamic therapy led to the eradication of amastigote from the lesions.
Healing and cosmetic outcome after photodynamic therapy was excellent. Only mild local inflammatory reaction was noted with no scarring. Then 1 and 4 months after the last photodynamic therapy session, there were no clinical signs of recurrence (Figures 1–4).
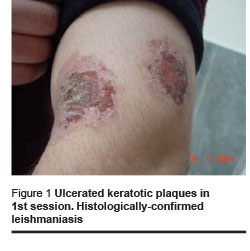
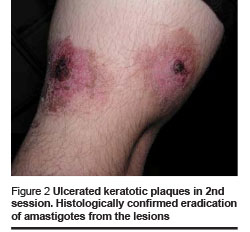
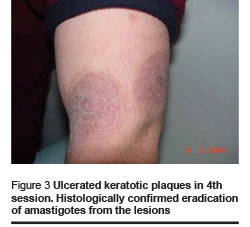
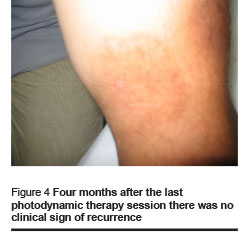
We found the following effects: tissue remodeling including flattening of the lesion and borders and filling of the ulcerated centre; re-epithelialization and development of epithelial tissue; reduction of tissue inflammation and post-inflammatory hyperpigmentation (Figures 1–4, Table 1).
Discussion
Treatment of cutaneous leishmaniasis is directed toward the eradication of amastigotes and the reduction of the size of the lesions to promote healing and achieve maximum efficacy with minimal scarring and toxicity [24], but no treatment has proved to be completely satisfactory. However, healing and cosmetic outcome after photodynamic therapy among our patients was excellent
The basis of this treatment is deficiency of enzymes in Leishmania [25,26]. Early in vitro investigations showed that porphyrins in combination with menadione induced selective destruction of amastigotes in the macrophages [27]. The antimicrobial activity of porphyrins might be related to their ability to catalyse different light-dependent chemical reactions [28]. Photodynamic therapy involves the administration of a photosensitizing compound and the selective accumulation of the sensitizer molecule in the target lesion, followed by irradiation of the lesion with visible light. The mechanism of preferential intralesional uptake of precursors and photosensitizing compounds with the light of an appropriate wavelength causes the destruction of tissue by the generation of reactive oxygen species and consequent peroxidation of lipids and cross-linking of proteins, which lead to severe interference with normal cellular functions.
In our patients, the brick-red fluorescence of the ALA-treated Leishmania lesions reflected a selective uptake and accumulation of high amounts of porphyrins after photo-cure application. During the irradiation with red light, the porphyrin-enriched tissues led to cell death. Results suggest that porphyrins represent effective molecules in treating cutaneous leishmaniasis by photodynamic therapy.
While our study only included 5 patients and we made no comparison with other treatments, the outcome among our patients was excellent. Photodynamic therapy appears to be an attractive antiparasitic therapeutic option that offers rapid localized destruction of the lesions without affecting the adjacent normal tissues. Furthermore, in contrast to systemic treatments, photodynamic therapy has no risk of toxicity. Thus photodynamic therapy might offer a promising treatment for disseminated lesions of cutaneous leishmaniasis. Mores studies are warranted with larger samples with comparison with other treatments.
Acknowledgements
We would like to thank Dr Y. Fathollahi, Dr A. Dalimi, Dr M. Nassiri, Dr Z.M. Hassan, Dr M. Sadri and Miss S. Ghasemi for reviewing the manuscript and providing their useful suggestions.
References
- Hepburn NC. Cutaneous leishmaniasis. Clinical and experimental dermatology, 2000, 25:363–70.
- Grevelink SA. Lerner EA. Leishmaniasis. Journal of the American Academy of Dermatology, 1996, 34:257–72.
- Bermarn JD. Human leishmaniasis: clinical, diagnostic and chemotherapeutic developments in the last 10 years. Clinical infectious diseases, 1997, 24:684–703.
- Gallis, HA. Amphotericin B: A commentary on its role as an antifungal agent and as a comparative agent in clinical trials. Clinical infectious diseases, 1996, 22(suppl. 2):S145–7.
- Nguyen MT et al. Orally administered amphotericin B in the treatment of oral candidiasis in HIV-infected patients caused by azole-resistant Candida albicans. AIDS, 1996, 10(14):1745–7.
- Graybill JR. Lipid formulations for amphotericin B: does the emperor need new clothes? Annals of internal medicine, 1996, 124(10):921–3.
- Soto JL et al. Comparison of generic to branded pentavalent antimony for treatment of new world cutaneous leishmaniasis. American journal of tropical medicine and hygiene, 2004, 71:577–81.
- Moskowitz PF, Kurban AK. Treatment of cutaneous leishmaniasis: retrospectives and advances for the 21st century. Clinical dermatology, 1999, 17:305–15.
- Davidson RN. Practical guide for the treatment of leishmaniasis. Drugs, 1998, 56:1009–18.
- Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nature reviews. Cancer, 2003, 3(5):380–7.
- Wilson BC. Photodynamic therapy for cancer: Principles. Canadian journal of gastroenterology, 2002, 16(6):393–6.
- Vrouenraets MB et al. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer research, 2003, 23:505–22.
- Dougherty TJ et al. Photodynamic therapy. Journal of the National Cancer Institute, 1998, 90(12):889–905.
- Dickson EFG, Goyan RL, Pottier RH. New directions in photodynamic therapy. Cellular and molecular biology, 2003, 48(8):939–54.
- Roberts DJH, Cairnduff F. Photodynamic therapy of primary skin cancer: A review. British journal of plastic surgery, 1995, 48:360–70.
- Peng Q et al. 5–aminolaevulinic acid-based photodynamic therapy. Cancer, 1997, 79:2282–308.
- Allison RR, Mang TS, Dale BD. Photodynamic therapy for the treatment of non-melanomatous cutaneous malignancies. Seminars in cutaneous medicine and surgery, 1998, 17(2):153–63.
- Colussi VC, Feyes DK, Mukhtar H. Perspectives of photodynamic therapy for skin diseases. Skin pharmacology and applied skin physiology, 1998, 11:336–46.
- Ruiz-Rodriguez R, Sanz-Sanchez T, Cordoba S. Photodynamic photorejuvenation. Dermatological surgery, 2002, 28:742–4.
- Goldberg DJ, Cutler KB. Nonablative treatment of rhytids with intense pulsed light. Lasers in surgery and medicine, 2000, 26:196–200.
- Bitter P Jr. Non-invasive rejuvenation of photo-damaged skin using serial, full-face intense pulsed light treatments. Dermatologic surgery, 2000, 26:835–43.
- Gardlo K et al. Treatment of cutaneous leishmaniasis by photodynamic therapy. Journal of the American Academy of Dermatology, 2003, 48(6):893–6.
- Enk CD et al. Treatment of cutaneous leishmaniasis with photodynamic therapy. Archives of dermatology, 2003, 139(4):432–4.
- Hewaldt BL. Leishmaniasis. Lancet, 1999, 354:1191–9.
- Chang KP, Chang CS, Sassa S. Heme biosynthesis in bacterium-protozoon symbioses: enzymic defects in host hemoflagellates and complemental role of their intracellular symbiotes. Proceedings of the National Academy of Sciences of the United States of America, 1975, 72:2979–83.
- Sah JF et al. Genetic rescue of leishmania deficiency in porphyrin biosynthesis creates mutants: Suitable for analysis of cellular events in uroporphyria and for photodynamic therapy. Biological chemistry, 2002, 277(17):14902–9.
- Abok K, Fredriksson BA, Brunk U. An experimental model system for leishmaniasis: effects of porphyrin compounds and menadione on Leishmania parasites engulfed by cultured macrophages. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica, 1988, 96:543–51.
- Stojiljkvic I, Evavold BD, Kumar V. Antimicrobial properties of porphyrins. Expert opinion on investigational drugs, 2001, 10:309–20.


