R.A.K. Mahmoud1 and M. Abdel Raouf2
ABSTRACT We evaluated the prognostic value of serum endostatin and vascular endothelial growth factor (VEGF) for diagnosis of pre-eclampsia. We determined VEGF and endostatin levels in the sera of 20 healthy, non-pregnant women and 64 pregnant women: 20 healthy, 20 with mild pre-eclampsia and 24 with severe pre-eclampsia. Serum levels of these factors in non-pregnant women were similar to those in healthy pregnant women. However, serum levels were significantly higher with mild or severe pre-eclampsia compared with normal pregnancies and significantly higher with severe rather than with mild pre-eclampsia. Elevated levels significantly increased risk more than severity of pre-eclampsia. VEGF and endostatin could be used to differentiate between pre-eclamptic and normal pregnancies and to discriminate mild pre-eclampsia from severe pre-eclampsia.
Concentration sérique d’endostatine et de facteur de croissance endothéliale vasculaire chez des patientes prééclamptiques
RÉSUMÉ Nous avons évalué la valeur pronostique de l’endostatine et du facteur de croissance endothéliale vasculaire (VEGF) dans le sérum pour le diagnostic de la prééclampsie. Nous avons déterminé la concentration d’endostatine et de VEGF dans le sérum de 20 femmes non enceintes en bonne santé et de 64 femmes enceintes : 20 en bonne santé, 20 ayant une prééclampsie bénigne et 24 une prééclampsie grave. La concentration sérique pour ces facteurs chez les femmes non enceintes était similaire à celle des femmes enceintes en bonne santé. Toutefois, la concentration sérique était significativement plus élevée en présence d’une prééclampsie bénigne ou grave par rapport aux grossesses normales et significativement plus élevée pour les prééclampsies graves par rapport aux prééclampsies bénignes. Une élévation de la concentration augmentait le risque significativement plus que la gravité de la prééclampsie. Le VEGF et l’endostatine pourraient être utilisés pour différencier les grossesses prééclamptiques et normales et pour établir une distinction entre une prééclampsie bénigne et grave.
1Department of Medical Biochemistry; 2Department of Obstetrics and Gynaecology, Faculty of Medicine, Cairo University, Cairo, Egypt (Correspondence to R.A.K. Mahmoud:
Received: 20/10/04; accepted: 10/01/05
EMHJ, 2006, 12(1-2): 178-187
Introduction
Pre-eclampsia is a multi-system disorder peculiar to human pregnancy and characte-rized by hypertension and proteinuria after 20 weeks of pregnancy. It remains a leading cause of maternal and neonatal mortality and morbidity [1].
Vascular endothelial growth factor (VEGF) plays a crucial role in physiological vasculogenesis and vascular permeability and has been implicated in the pathogenesis of pre-eclampsia. It interacts with 2 tyrosine kinases: fms-like tyrosine kinase-1, Flt-1 (VEGFR-1), and kinase domain-containing region, Flk-1/KDR (VEGFR-2) [2]. Vascular growth during implantation and placentation is critical for successful gestation. It is thought that vascular insufficiencies during placentation contribute to pre-eclampsia [3]. Oxygen tension and cytokines have been shown to influence trophoblast VEGF expression, suggesting that this particular family of angiogenic proteins plays an important role in placental angiogenesis [3].
Pre-eclampsia occurs in 2 phases: abnormal implantation of the placenta leads to impaired placental blood flow, which then induces the release of a critical placental substance into the maternal circulation. Despite considerable research, the cause or causes of pre-eclampsia remain unclear, and there are no clinically useful screening tests to identify women in whom it will develop [4].
Endostatin is derived from the noncollagenous domain 1 (NC1) at the C-terminus of collagen type XVIII. It inhibits endothelial cell proliferation and migration [5]. It is an abundant proteoglycan in vascular basement membranes and the walls of major blood vessels that maintain vascular permeability; its loss enhances angioge-nesis and vascular permeability [6]. To our knowledge, few reports have evaluated the potential role of endostatin and VEGF in pre-eclampsia [7].
We investigated the relationship of VEGF as an angiogenic growth factor and endostatin as an angiogenic inhibitor in patients with pre-eclampsia. The possible association between serum levels of these proteins and risk of pre-eclampsia and correlations between them were analysed.
Methods
This study was conducted between January and June 2004 with 84 subjects (20 normal non-pregnant controls, 20 normal pregnant controls, 20 patients with mild pre-eclampsia, and 24 patients with severe pre-eclampsia). All subjects were recruited from the Obstetrics and Gynaecology Outpatient Clinic, Kasr Al-Aini Hospital, Cairo University, Cairo, Egypt. Demographic and clinical data were collected during routine obstetric visits.
Pre-eclampsia was diagnosed according to the American College of Obstetricians and Gynecologists (ACOG) criteria [8]: presentation after 20 weeks gestation with sustained and elevated blood pressure (BP) in a previously normotensive patient, with the mild form defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg and the severe form defined as systolic BP ≥ 160 mmHg and/or diastolic BP ≥ 110 mmHg; in addition, the presence of proteinuria excretion > 5 g in 24-hour urine. Exclusion criteria were a history of essential hypertension, multifetal gestation, diabetes, chronic renal disease, miscarriage, ante-partum haemorrhage, platelet disorders, maternal or fetal infection, autoimmune disorders and epilepsy.
For our control groups, we recruited healthy pregnant women also attending the clinic for routine examinations during pregnancy and non-pregnant women attending the gynaecological clinic. Controls had no history of illness, especially no forms of hypertension or renal disease. All controls were matched with the pregnant women for age, gestational age (32–41 weeks) and parity.
Blood samples were drawn at initial presentation to the clinic. No subjects had received antihypertensive medication, steroids, prostaglandins or magnesium sulfate before blood sampling. No one was in labour when the blood sample was drawn. Peripheral venous blood was collected from all subjects, allowed to clot at room temperature, centrifuged at 3000 rpm for 20 minutes and then stored at –80 °C in multiple aliquots for analysis. Serum levels of VEGF and endostatin were determined with in vivo enzyme immunoassay for the detection of total human VEGF in complex biological fluids and in vivo enzyme immunoassay for the detection of total human endostatin protein in complex biological fluids respectively (Accucyte Human VEGF and Endostatin, Cytimmune Sciences Inc., College Park, Maryland, United States of America). Each assay was performed according to the manufacturer’s instructions.
Statistical analyses were performed with SPSS, version 11. Numerical data were expressed as mean (standard deviation). Chi-squared test was used to examine the relationship between qualitative variables. For quantitative data, the Kruskal–Wallis test was used for comparison between 4 groups. The Mann–Whitney test was used to compare between 2 groups. Relationships between numerical variables were tested with Spearman correlation analysis. Multivariate regression analysis was applied on significant variables on univariate analysis. Predictive effects of VEGF and endostatin levels on occurrence and seve-
rity of pre-eclampsia were investigated with univariate logistic regression analyses on the entire pregnant sample. The results are presented as odds ratios with 95% confidence intervals.
Receiver operating characteristic curves (ROC) were plotted to identify the cut-off values of the 2 variables to differentiate between normal pregnant and pre-eclamptic women, and between mild pre-eclampsia and severe pre-eclampsia. Comparisons and correlations were statistically significant when P < 0.05.
Results
Table 1 shows mean age, gestational age at sampling and clinical characteristics of the study groups, e.g. blood pressure and proteinuria. As the women were matched for age and gestational age there was no significant difference among the groups of women regarding these variables. Pregnant women with pre-eclampsia had significantly higher systolic blood pressure, dystolic blood pressure and proteinuria than normal controls.
Women with mild or severe pre-eclampsia had significantly higher elevated serum VEGF and endostatin levels than healthy pregnant or non-pregnant controls. VEGF and endostatin levels were higher with severe pre-eclampsia (Table 2). However, the VEGF/endostatin ratio was significantly higher with severe pre-eclampsia only as compared with the normal pregnant women or mild pre-eclampsia. There was a significant positive correlation between serum VEGF and endostatin levels (r = 0.70, P < 0.001) and between each of serum VEGF and endostatin levels and each of systolic blood pressure, diastolic blood pressure and proteinuria respectively (for VEGF, r = 0.80, 0.75, and 0.76, P < 0.001 respectively, and for endostatin, r = 0.78, 0.66, and 0.68, P < 0.001 respectively). Neither VEGF nor endostatin were significantly correlated with age or gestational age in pre-eclamptic patients. Multivariate regression analysis revealed similar significant correlations with the exception that diastolic BP and endostatin became no more significant.
The sample of pregnant women was investigated as a unit by univariate logistic regression analyses (Table 3). When a subject had a high VEGF or endostatin levels, she had elevated odds (approximated risk) of pre-eclampsia. VEGF and endostatin, respectively, demonstrated 1.54 fold and 1.24 fold increase in risk for pre-eclampsia for every 1 ng/mL increase in serum levels compared with normal pregnancies. The risk for severe pre-eclampsia increased 1.07 fold and 1.09 fold respectively compared with mild pre-eclampsia.
Different cut-off values were used to determine the sensitivity and the specificity for VEGF and endostatin as possible predictor markers of pre-eclampsia. A VEGF cut-off value of 29 ng/mL discriminated pre-eclamptic pregnancies from normal pregnancies very well, with sensitivity of 100%, specificity of 87.5% and the area under the curve at 0.99. A cut-off value for endostatin level at 21 ng/mL discriminated pre-eclamptic from normal pregnancies well, with sensitivity of 86.4%, specificity of 82.5% and the area under the curve at 0.93. Cut-off values of 65 ng/mL and 42 ng/mL respectively, for VEGF and endostatin discriminated severe pre-eclampsia from mild pre-eclampsia with sensitivity of 95.8% and 75%, specificity of 90% and 70%, and areas under the curves at 0.94 and 0.81 respectively (Figures 1 and 2).
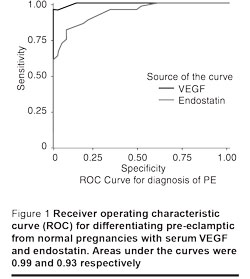
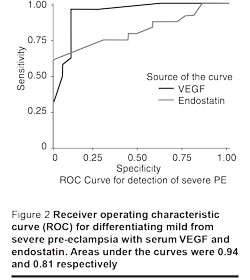
Discussion
Serum VEGF and endostatin were elevated with pre-eclampsia compared with normal pregnancies and were much higher with severe than with mild pre-eclampsia. VEGF and endostatin appear to be useful markers for the detection of pre-eclampsia and its severity.
VEGF levels are elevated in pre-eclamptic sera after the onset of the disease. The excess protein might be derived from the placenta, maternal platelets, or smooth muscle cells. Its elevation might contribute to the extravasation of plasma proteins, the development of proteinuria, decreased endothelium-dependent vascular dilatation, enhanced basal tone and permeability and signs of endothelial dysfunction [1,9].
Several researchers have reported increased systemic VEGF in women with pre-eclampsia although others have reported decreased VEGF or no change [4,10–15]. These discrepancies may reflect the variety of assays used and the different physical states of the cytokine (bound or free) [16].
VEGF had high sensitivity and specificity. It may be a clinically useful biomarker to more accurately diagnose pre-eclampsia and to differentiate between mild and severe forms [11]. Serum VEGF levels are significantly elevated in clinical pre-eclampsia and fall to levels similar to those of normal patients within 24 hours of delivery [12]. This suggests that the main source of VEGF production is with the fetus and the placenta. That VEGF was not elevated in pregnancy was surprising because it is involved in both embryogenesis and placental formation [14,15].
Normally Flt-1 positively regulates sprout formation by modulating signalling through Flk-1 and by the production of a soluble form of its receptor (sFlt-1) that antagonizes VEGF signalling [17]. Perhaps this is a protective response by the placenta to mop up the excess serum VEGF levels circulating in maternal blood [12]. Continuous low levels of VEGF are required for endothelial cells to survive prolonged periods and function properly [18]. Factors in addition to sFlt1 are likely to play a role in the pathogenesis of pre-eclampsia as a subset of patients with pre-eclampsia had only slightly elevated serum sFlt1 levels. Thus, sFlt1 may be one of several factors produced by the placenta to influence the severity of pre-eclampsia [18].
We suggest that elevated endostatin levels represent part of a defence mechanism to protect the host from tumour angiogenesis as the host reacts to the invasion of trophoblasts as a malignant condition. Human placental development does combine elements of tumourigenesis and vasculogenesis [19]. In patients with non-Hodgkin lymphoma, elevated endostatin levels were significantly associated with elevated VEGF levels, suggesting that coupling between the angiogenesis-promoting and the angiogenesis-inhibiting factors is to control the rate of tumour angiogenesis or is simply a by-product of proteases [21].
VEGF promotes the secretion of matrix metalloproteinase (MMP) from endothelial cells to allow for migration of endothelial cells [22]. However, MMPs might cleave endostatin from collagen XVIII during sprouting angiogenesis, which contributes to endostatin increase in the sera of patients with pre-eclampsia. Elevated concentrations of endostatin could play a role in the pathophysiology of pre-eclampsia by counteracting the effects of VEGF [7]. However, endostatin in healthy pregnant women was not greater than in non-pregnant women in either our study or the 2003 Hirtenlehner et al. study, suggesting that villous basement membranes of the placenta might not contribute to endostatin production [7].
In pre-eclampsia, increased serum endostatin could be derived from different maternal sources, such as platelets or blood vessels that have the capacity to produce proangiogenic and antiangiogenic factors under certain conditions. The potential hypoxic or inflammatory conditions of pre-eclampsia might also induce the release of the inhibitor from the placenta [23]. Elevated endostatin in the sera of pre-eclamptic women suggests that the expression of an antiangiogenic factor is disturbed in pre-eclampsia [7].
Altered serum concentrations of VEGF and endostatin, which may exhibit antagonistic functions through binding to the KDR/Flk-1 receptor, could play a critical role in the condition. Endostatin blocked VEGF-induced tyrosine phosphorylation of KDR/Flk-1 and its downstream signalling events, inhibiting VEGF-induced endothelial cell proliferation and migration [24]. Endostatin blocks VEGF-induced nitric oxide synthesis and thereby inhibits VEGF-induced endothelial cells migration and angiogenesis [25].
We found significant positive correlations between both VEGF and endostatin with blood pressure. The antimigratory effect of endostatin involves impairment of cell-matrix interactions; it interacts with integrin at the endothelial cell surface [26]. Low concentrations of free VEGF and nitric oxide and increased levels of total VEGF and sFlt-1 in the second trimester may represent an impaired stimulus to vascular formation and endothelial regulation that induce placental disease and pre-eclampsia [18]. Thus, endostatin may further aggravate the adverse effects of an unfavourable balance of VEGF and its antagonist. Furthermore, it can bind to Flt-1 and block the interaction between VEGF and Flt-1 [24]. Endostatin induces apoptosis and reduces cell proliferation and migration of endothelial cells through inhibition of cyclin D1 and Wnt signalling, respectively [5].
Mice deficient for Wnt receptors and its signalling display vascular abnormalities, including defective placental vasculature, and are embryonic lethal [27]. The endogenous endostatin down-regulates many signalling pathways in human microvascular endothelium associated with proangiogenic activity and simultaneously up-regulates many antiangiogenic genes [28].
The demand for VEGF signalling in the glomerulus is much higher than in other tissues, probably for the maintenance of the glomerular filtration barrier. A critical threshold is required to maintain the established vasculature in the adult. Reduction or overexpression of VEGF or increase of sFlt1 develop into glomerular endotheliosis, hypertension and proteinuria, i.e. the renal lesion seen in pre-eclampsia) [29]. We found positive correlations between both VEGF and endostatin with proteinuria.
Maynard et al. suggested important implications for the use of VEGF inhibitors in the therapy of pre-eclampsia and other diseases such as cancer that pose a risk of endothelial dysfunction, renal insufficiency and hypertension [18]. Therapeutic strategies using exogenous VEGF to shift the angiogenesis balance in favour of proangiogenic molecules might reverse the endothelial dysfunction seen in pre-eclampsia patients and allow delivery to be safely postponed [18].
Elevated endostatin concentration could be a major component of the pre-eclamptic plasma, impairing endothelial cell growth and contributing to the occurrence of endothelial lesions in pre-eclampsia. It might also be disadvantageous to specific developmental processes of the placenta such as angiogenesis, branching of villi, or trophoblast differentiation [7]. The increase in endostatin by 1 ng/mL significantly increased the odds of mild pre-eclampsia by 16.4% (odds ratio 1.164) and the odds of severe pre-eclampsia by 18.1% (odds ratio 1.181) compared with normal pregnancies. Endostatin did not have significant effects on the severity of pre-eclampsia [7].
We found that VEGF and endostatin increased significantly the odds of pre-eclampsia more than the severity of pre-eclampsia. However, the relative risk associated with VEGF was greater than the relative risk associated with serum endostatin. Indeed, the imbalance between stimulators and inhibitors of angiogenesis might be involved in the pathogenesis of several disorders in which vascular dysfunction is a contributing factor, e.g. in patients with different cancers or endometriosis [30,31]. Similar to our results, endostatin and VEGF had a significant positive correlation in patients with non-Hodgkin lymphoma [20]. In our study, the VEGF to endostatin ratio was significantly higher in severe pre-eclampsia than in mild pre-eclampsia or in normal pregnancies.
According to both odds ratios and ROC analyses, VEGF and endostatin are potentially useful tools for the early identification of patients at risk of pre-eclampsia. Prospective studies are needed to establish the clinical value of monitoring endostatin levels in pre-eclamptic patients throughout pregnancy. In addition, measuring its concentration in conjunction with other soluble angiogenesis-regulating mediators’ sFlt1 may aid the angiogenic profiling of patients with pre-eclampsia.
Conclusion
Our data suggest that many factors participate in the pathogenesis of pre-eclampsia. These include elevated levels of VEGF and endostatin with subsequent alterations in angiogenesis. VEGF and endostatin may be used as clinical biomarkers for pre-eclampsia and to differentiate between mild and severe pre-eclampsia; serum VEGF, however, is more sensitive and specific than endostatin.
References
- VanWijk M et al. Vascular function in pre-eclampsia. Cardiovascular research, 2000, 47(1):38–48.
- Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocrine reviews, 2004, 25(4):581–611.
- Torry D, Hinrichs M, Torry R. Determinants of placental vascularity. American journal of reproductive immunology, 2004, 51(4):257–68.
- Levine R et al. Circulating angiogenic factors and the risk of pre-eclampsia. New England journal of medicine, 2004, 350(7):672–83.
- Hanai J et al. Endostatin causes G1 arrest of endothelial cells through inhibition of cyclin D1. Journal of biological chemistry, 2002, 277(19):164–9.
- Moulton K et al. Loss of collagen XVIII enhances neovascularization and vascular permeability in atherosclerosis. Circulation, 2004, 110(10):1330–6.
- Hirtenlehner K et al. Elevated serum concentrations of the angiogenesis inhibitor endostatin in pre-eclamptic women. Journal of the Society for Gynecological Investigation, 2003, 10:412–7.
- American College of Obstetricians Gynecologists. ACOG practice bulletin. Diagnosis and management of pre-eclampsia and eclampsia. International journal of gynaecology and obstetrics, 2002, 77(1):67–75.
- Svedas E et al. Vascular endothelial growth factor induced functional and morphologic signs of endothelial dysfunction in isolated arteries from normal pregnant women. American journal of obstetrics and gynecology, 2003, 188:168–76.
- Kupferminc M et al. Vascular endothelial growth factor is increased in patients with pre-eclampsia. American journal of reproductive immunology, 1997, 38:302–6.
- El-Salahy E et al. New scope in angiogenesis: Role of vascular endothelial growth factor (VEGF), NO, lipid peroxidation, and vitamin E in the pathophysiology of pre-eclampsia among Egyptian females. Clinical biochemistry, 2001, 34:323–9.
- Hunter A et al. Serum levels of vascular endothelial growth factor in preeclamptic and normotensive pregnancy. Hypertension, 2000, 36:965–9.
- Bielecki D et al. Niektore czynniki wzrostu w ciazy powiklanej preeklampsja. [Growth factors in pregnancy complications with pre-eclampsia.] Ginekologia polska, 2002, 73(5):422–9.
- Livingston J et al. Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe pre-eclampsia. American journal of obstetrics and gynecology, 2000, 183(6):1554–7.
- Reuvekamp, A. et al. Selective deficit of angiogenic growth factors characterizes pregnancies complicated by pre-eclampsia. British journal of obstetrics and gynaecology, 1999, 106:1019–22.
- Anthony F et al. Variation in detection of VEGF in maternal serum by immunoassay and the possible influence of binding proteins. Annals of clinical biochemistry, 1997, 34:276–80.
- Roberts D et al. The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signalling during blood vessel formation. American journal of pathology, 2004, 164:1531–5.
- Maynard S et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension and proteinuria in pre-eclampsia. Journal of clinical investigation, 2003, 111:649–58.
- Zhou Y et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe pre-eclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. American journal of pathology, 2002, 160:1405–23.
- Bono P, Teerenhovi L, Joensuu H. Elevated serum endostatin is associated with poor outcome in patients with non-hodgkin lymphoma. Cancer, 2003, 97:2767–75.
- Ferreras M et al. Generation and degradation of human endostatin proteins by various proteinases. FEBS letters, 2000, 486:247–51.
- Narumiya H et al. Matrix metalloproteinase-2 is elevated in the plasma of women with pre-eclampsia. Hypertension in pregnancy, 2001, 20(2):185–94.
- Ergun S et al. Endostatin inhibits angiogenesis by stabilization of newly formed endothelial tubes. Angiogenesis, 2001, 4:193–206.
- Kim Y et al. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. Journal of biological chemistry, 2002, 277(31):27872–9.
- Urbich C et al. Dephosphorylation of endothelial nitric oxide synthase contributes to the anti-angiogenic effects of endostatin. FASEB journal, 2002, 16(7):706–8.
- Wickstrom S, Alitalo K, Keski-Oja J. Endostatin associates with integrin alpha5beta1 and caveolin-1, and activates Src via a tyrosyl phosphatase-dependent pathway in human endothelial cells. Cancer research, 2002, 62(19):5580–9.
- Goodwin A, D’Amore P. Wnt signaling in the vasculature. Angiogenesis, 2002, 5(1–2):1–9.
- Abdollahi A et al. Endostatin’s antiangiogenic signaling network. Molecular cell, 2004, 13(5):649–63.
- Eremina V, Quaggin S. The role of VEGF-A in glomerular development and function. Current opinion in nephrology and hypertension, 2004, 13(1):9–15.
- Dhar D et al. Serum endostatin predicts tumour vascularity in hepatocellular carcinoma. Cancer, 2002, 95:2188–95.
- Liu M, He Y, Peng D. Expression of vascular endothelial growth factor and endostatin in peritoneal fluid of patients with endometriosis. Di yi jun yi da xue xue bao, 2004, 24(1):69–71.


