Ebrahim Rahimi,1 Rasool Mohammadi,2 Yaser Mokhayeri3 and Seyed S.H. Nazari4
1Department of Public Health, Mamasani Higher Education Complex for Health, Shiraz University of Medical Sciences, Shiraz, Islamic Republic of Iran. 2Department of Biostatistics and Epidemiology, School of Public Health and Nutrition, Lorestan University of Medical Sciences, Khorramabad, Islamic Republic of Iran. 3Cardiovascular Research Center, Shahid Rahimi Hospital, Lorestan University of Medical Sciences, Khorramabad, Islamic Republic of Iran. 4Prevention of Cardiovascular Disease Research Center, Department of Epidemiology, School of Public Health and Safety, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic of Iran. (Correspondence to Seyed S.H. Nazari:
Abstract
Background: Gender disparity in the risk of noncommunicable diseases and its relationship with other social health determinants is not well researched.
Aims: To assess the factors contributing to gender disparity in overall risk of noncommunicable diseases in the Islamic Republic of Iran.
Methods: This study was a secondary analysis of data on about 11 000 adults aged 15–69 years from the 2011 STEPS survey in the Islamic Republic of Iran. The outcome variable in our analysis was the noncommunicable disease risk factor index. We used an extension of the Blinder-Oaxaca decomposition model to decompose the predicted mean difference in this index. Sampling method and study design were taken into account in the analysis. As well as sex, the predictor variables were: age; household assets index; education; work status; ethnicity; and residence.
Results: The overall mean (standard deviation) noncommunicable diseases risk score was 39.26 (22.4). The risk score for women was significantly higher than that for men (41.75 versus 36.84; P < 0.001). About 35% of gender disparity in risk score was due to the different distribution of the predictor variables (explained component); of these, age contributed the most (23.79%), followed by education (7.82%). The different gender effects of work status and age made the largest contributions to the unexplained component of the disparity, 36.40% and 14.82%, respectively.
Conclusions: Policies to reduce the risk of noncommunicable diseases need to consider gender groups and how gender interacts with social determinants such as work status to make some gender subgroups more vulnerable than others.
Keywords: noncommunicable diseases; risk factors; gender equity; Iran.
Citation: Rahimi E; Mohammadi R; Mokhayeri Y; Nazari SSH. Decomposing gender disparity in the risk of noncommunicable diseases in adults, Islamic Republic of Iran. East Mediterr Health J. 2023;29(6). https://doi.org/10.26719/emhj.23.046
Received: 15/09/21; accepted: 21/11/22
Copyright: © Authors; licensee World Health Organization. EMHJ is an open access journal. All papers published in EMHJ are available under the Creative Commons Attribution Non-Commercial ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Introduction
Noncommunicable diseases (NCDs) are the leading cause of both morbidity and mortality worldwide (1). About 71% of all deaths and 43% of the world’s disease burden are attributed to NCDs; these percentages are higher in low- and middle-income countries, with 79% and 85% being reported, respectively (2). The Islamic Republic of Iran is also facing the challenge of NCDs: 83.5% of all deaths and 78.1% of the burden of diseases were attributed to NCDs in 2019 (1).
The rising prevalence of NCDs is caused by unhealthy lifestyles and behavioural changes, especially in developing countries (3). Determining the prevalence of the risk factors for these diseases, combined with effective policy-making for their management, can slow the rising trend (4). In most interventions aimed at promoting health, NCDs risk factors are considered in isolation. However, some NCDs, mainly cardiovascular disease, cancer, chronic respiratory disease and diabetes, share common risk factors. Therefore prevention and control of any of these diseases should integrated into approaches to prevent NCDs as a group. This integrated approach can maximize collaboration and improve efficiency of the prevention and control measures, which is especially important for efficient use of limited resources (5)
Given that these diseases and their risk factors vary by community and population groups (4), their disease burden differs in these groups. Ignoring cultural and social differences when designing control programmes will likely widen health gaps between the populations (2,6). Even though NCDs have received increased attention (7,8), the fact that the contextual conditions for acquiring such diseases are different for each community has been neglected (4,6).
Some studies have shown that social- and gender-related disparities exist in the risk of NCDs (4,9–12). These gender disparities in NCD risk and their relationship with other social health determinants, such as education, residence, employment and socioeconomic status, have not received explicit attention (13–15).
Given the evidence on the different prevalence of most NCDs and their risk factors in men and women in the Islamic Republic of Iran (16,17), this study aimed to assess the contribution of various factors to the gender disparity. Determining the origins of the disparity will help policy-makers develop more effective intervention programmes aimed at reducing the burden of NCDs (18).
Methods
Study design and sample
This study was a cross-sectional analysis of secondary data from the sixth round of the national STEPS survey in Islamic Republic of Iran in 2011. The nationwide survey (primary study) was a cross-sectional, population-based study based on the World Health Organization’s (WHO) STEPwise approach to surveillance of NCD risk factors (19). It included about 12 000 Iranian people aged 6–70 years selected through multistage cluster random sampling with inclusion probability proportional to the population size. In our study we included about 11 000 adults aged 15–69 years. To compensate for over-sampling in some provinces and to increase the accuracy of the estimations, the sampling method and study design were taken into account in the analysis. For this purpose, the data were weighted based on the population.
Outcome variable
The outcome variable was the NCD risk factor index as a proxy for NCD risk score. The risk factors considered for estimating risk score were: insufficient physical activity; low fruit and vegetable consumption; cigarette smoking; overweight and obesity; and high blood pressure. We included these core risk factors because they have the greatest impact on NCD burden, modification is possible through effective intervention and their information can be obtained using questionnaire and simple physical measurements (20). All risk factors were expressed as binary variables according to standard recommendations (21–24). Sufficient physical activity was considered a combination of moderate and vigorous intensity physical activity at least 600 metabolic equivalent of task (MET) minutes per week according to WHO recommendations (21). The general recommendation for daily intake of fruits and vegetables is five or more servings, and this was the cut-off used in our analysis (22). Current and daily use of any type of cigarette (factory-made cigarettes, hand-rolled cigarettes or cigars) was considered current cigarette smoking. Based on body mass index (BMI), participants with a BMI ≥ 25 kg/m2 were classified as overweight and obesity (23). High blood pressure was defined as a systolic blood pressure > 140 mmHg, a diastolic blood pressure > 90 mm Hg, or current use of antihypertensive medication (24).
To account for the different contribution of each risk factor to the burden of NCDs, scoring for each risk factor was weighted proportionate to its contribution (25). That means the presence of each risk factor was scored relative to its contribution to the burden of NCDs, and absence of that the risk factor resulted in a contribution of 0 to the final index. The total risk score for each respondent was calculated from the sum of his/her weighted scores for all risk factors. These scores were then converted into a 0–100 scale.
Predictor variables
In addition to sex (male/female), the potential predictor variables examined were: age (in years); household assets index (see below); highest educational level (no education, < high-school diploma, high-school diploma, higher education (at least some college)); work status (employed, unemployed, non-labour force according to the Bureau of Labour Statistics (26)); ethnicity (Persian/non-Persian) and residence (rural/urban). To determine the household assets index, factors related to the infrastructure and facilities of the home were measured, including access to a dedicated bathroom, kitchen, refrigerator, vacuum cleaner, washing machine and computer. Using polychoric principal component analysis and applying the correlation matrix, the variables with the greatest impact on the total variance were identified. Finally, the first principal component (new variable) that accounted for > 65% of the combined variance was considered the household assets index.
Statistical analysis
Stata, version 14 was used for all analyses. We used descriptive statistics, including central tendency and dispersion indices for quantitative variables and frequencies and percentages for qualitative variables. To decompose the gender disparity in the overall risk of NCDs, an updated package of the Blinder-Oaxaca decomposition model was used (27,28). The mean predicted gender difference in the NCD risk factor index from the model was considered an estimate of the related gender disparity. The model, which is based on a counterfactual regression approach, considers the frequency distribution and differential effect of contributing factors that vary across gender groups, i.e. it decomposes the gender disparity in the overall risk of NCDs into smaller components. Thus, the method explains how much of the disparity in mean predicted outcome (NCD risk factor index) is due to differences in the levels of the related factors in males and females (explained components) and how much is due to gender discrimination, but may also be due the differential effect (the magnitude of regression coefficients) of the factors and other unknown associated factors (unexplained components). In fact, the existence of inequality despite identical individual characteristics can be rooted in unknown factors that affect the outcomes (27,29).
Assuming that the NCD risk score (Y) is explained by n predictors (x1, ... .xn), the gender mean difference in predicted Y(D) is as follows:
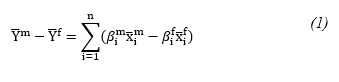
Where x ̅i is the mean value of each predictor variable and β is the predicted regression coefficient.
If the male coefficients are used as the reference, then decomposition is formulated from the viewpoint of females. In other words, the counterfactual equation (βim xi^f) can be obtained by replacing the female coefficients with those of the male equation. By adding and subtracting the counterfactual equation in the right hand side of equation (1), the gender disparity in the mean predicted Y(D) can be decomposed into explained (E) and unexplained (U) components. It is straightforward to show that:
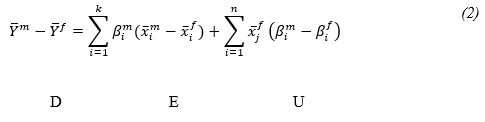
Ethical approval
Approval for the study was obtained from the ethics committee of the Shahid Beheshti University of Medical Sciences. Tehran (IR.SUMS.RETECH.REC.1399.765).
Results
Baseline characteristics
The baseline characteristics of all study participants is given in Table 1. Men comprised about 51.86% of the participants, and most (68.53%) were urban residents. The mean age (standard deviation (SD)) of the participants was 35.89 (SD 14.37) years. In terms of education, 16.71% were not educated, while 46.11% had graduated from high school or had a higher education. Less than half the participants (42.88%) of were part of the labour force, while. 28.45% of men and 86.68% of women were non-labour force. Almost half of the participants (47.94%) were of Persian ethnicity. Significant differences were found between males and females for: age (P= 0.001), household assets index (P= 0.001), educational attainment (P= 0.001), work status (P < 0.001) and type of work (P = 0.001). The mean NCD risk factor index was significantly higher in women (41.75; SD 24.31) than men (36.84; SD 18.78) (P < 0.001).
Low consumption of fruits and vegetables (73.58%) was the leading risk factor, followed by obesity and overweight (49.23%), insufficient physical activity (38.68%), high blood pressure (22.05%) and cigarette smoking (11.53%). Significant gender differences were found in cigarette smoking (men 21.76%; women 1.01%), insufficient physical activity (men 26.73%; women 50.99% and obesity and overweight (men 43.05%; women 55.59%); all P = 0.001.
Decomposition results
The decomposition of the gender disparity in predicted mean NCD risk is shown in Table 2. The disparity was in favour of men (P < 0.001). Only about 35% of the gender disparity was due to the different distribution of the associated factors (explained component). Age contributed the most (23.79%) to the explained component, followed by educational attainment (7.82%). About two thirds of the disparity was attributed to the different effect of the variables (unexplained component), of which about 20.96% was due to unknown variables that were not included in the model. The different effects of work status and age made the largest contribution to the unexplained component, accounting for 36.40 and 14.82%, respectively.
Discussion
We aimed to assess the contribution of related factors to gender disparity in the overall risk of acquiring NCDs in adults living in the Islamic Republic of Iran using data from a nationwide survey. Our findings suggest that men and women have different levels of exposure to NCD risk factors. Moreover, the overall NCD risk for women was higher than for males. Decomposition of the risk showed that work status, educational level and age substantially contributed to this gender difference.
Consistent with other study (12,17,30,31), our study highlights the higher risk of NCDs in women. Evidence from a new WHO report shows that the different gender roles and cultural norms have a major effect on the NCD risks of men and women. These factors tend to change with age and according to social and cultural influences. Accordingly, this leads to different behaviours which affect exposure to NCD risk factors and health and help-seeking behaviour (32).
Despite this higher risk, the focus on women’s health is often limited to reproductive ages and NCDs are considered primarily men’s diseases (12,33). This gender bias could lead to inadequate screening of NCD risk factors in women, making it difficult to control these diseases in women (12). While women tend to live longer than men, older women experience poorer health and more and NCD multimorbidity than older men, with higher associated cost (12,33). This highlights a challenge in health care provision for older women in the Islamic Republic of Iran.
Our findings suggest that the mean age difference between males and females contributed the most to the disparity in NCD risk factor index. In addition, our study indicates that a significant part of the observed disparity was attributed to the different gender effects of age. That means the women experienced greater NCD risk with increasing age than men. This finding is consistent with other studies suggesting multimorbidity is more prevalent in elderly women than in elderly men (16,17,34,35). Of course greater longevity is associated with increased morbidity and women tend to live longer than men. At the same time, elderly women often encounter multiple challenges including financial insecurity, illiteracy, discrimination, domestic violence, physical dependency and a greater possibility of living alone. As well as these difficulties, their health needs and specific requirements are often neglected (33). Therefore, more attention should be given to elderly women in this regard.
Work status also contributed to the observed disparity in our study. This may be due to different jobs and working conditions between men and women in the labour market, which in turn has resulted in different prevalence rates and patterns of health outcomes in men and women (15). According to the Framingham Study, the combination of occupation and family responsibility roles exerted considerable pressure on women. Interpersonal relationships, coping styles and occupations of some working women, along with family responsibilities, have been reported to influence the development of some NCDs in women, such as coronary heart disease (36). This finding is plausible given that the activities generally expected of women in Iranian society involve less physical activity than those associated with men, which is in line with other studies in the Islamic Republic of Iran (29,37). In a gender-biased society, women often have less access to education and social and health services, and fewer employment opportunities than men. Moreover, in Iranian society, education and work settings are also highly gendered which could cause gender inequalities in health. In spite of the substantial increase in women’s enrolment in higher education in the Islamic Republic of Iran, significant development in the women’s labour force has not taken place and they continue to face discrimination in the job opportunities (38).
Furthermore, different gender effects of educational status also contributed to the disparity. Thus, equalizing the educational levels of men and women could potentially reduce the total difference. This finding, together with female educational levels below those of men, suggests that the overall risk of NCDs for women tends to be lower in more highly educated women. This corresponds with study in the Republic of Korea that showed a higher burden of NCDs in less educated groups, especially in women (39); however, it was not the case in an earlier study among Asians (40).
Data for our study were taken from the sixth round of the national STEPS survey in Islamic Republic of Iran, a population-based survey based on the STEPwise approach proposed by WHO (20) and adapted to local conditions. Given the study design, our findings could be generalized to the entire population. However, despite adopting a suitable approach in measuring and explaining gender disparity in NCD risk factors and taking into account the roles of the related social determinants of health, our study had some limitations. First, we used data from a national survey and could not therefore ascertain the reliability of the data. Furthermore, as with other cross-sectional studies, causal relationships between the variables cannot be determined. Therefore, our findings should be used with caution if considering policy-making and intervention design. Future research should focus on determining whether or not the identified correlations are causal in nature, thereby providing the knowledge required for effective control and prevention of NCD risks, especially in women.
Conclusions
Our findings highlight the importance of gender-based population level interventions aimed at preventing NCDs. NCD risk-reduction policies need to consider not only gender groups but also how gender interacts with social determinants to make some gender subgroups more vulnerable than others. Elderly women should be a focus of interventions aimed at reducing gender disparity in the risk of common NCDs. Targeted education about healthy lifestyles in these women and improving their knowledge of NCD risk factors can reduce the risk of these diseases. Women’s occupation is also a fundamental factor in NCD risk. Therefore a women’s information registration system and the use of appropriate key indicators to periodically monitor and evaluate their health is recommended. To this end, further cooperation between government agencies, research institutes and academia related to women’s health issues seems necessary.
Acknowledgements: The authors thank the centre for NCD control of the Iranian Ministry of Health and Medical Education for providing the data for this study.
Funding: None.
Competing interests: None declared.
References
- Azadnajafabad S, Mohammadi E, Aminorroaya A, Fattahi N, Rezaei S, Haghshenas R, et al. Non-communicable diseases’ risk factors in Iran; a review of the present status and action plans. J Diabetes Metab Disord. 2021:1–9. https://doi.org/10.1007/s40200-020-00709-8
- Noncommunicable diseases. Key facts [Internet]. Geneva: World Health Organization (https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases, accessed March 2022).
- Di Cesare M, Khang YH, Asaria P, Blakely T, Cowan MJ, Farzadfar F, et al. Inequalities in non-communicable diseases and effective responses. Lancet. 2013;381(9866):585–97. https://doi.org/10.1016/S0140-6736(12)61851-0
- Allen L, Williams J, Townsend N, Mikkelsen B, Roberts N, Foster C, et al. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: a systematic review. Lancet Glob Health. 2017;5(3):e277–e89. https://doi.org/10.1016/S2214-109X(17)30058-X
- Habib SH, Saha S. Burden of non-communicable disease: global overview. Diabetes Metab Syndr. 2010;4(1):41–7. https://doi.org/10.1016/j.dsx.2008.04.005
- Mathers C, Vos T, Stevenson C. The burden of disease and injury in Australia. Canberra: Australian Institute of Health and Welfare; 1999.
- Daar AS, Singer PA, Persad DL, Pramming SK, Matthews DR, Beaglehole R, et al. Grand challenges in chronic non-communicable diseases. Nature. 2007;450(7169):494–6. https://doi.org/10.1038/450494a
- Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, et al. Priority actions for the non-communicable disease crisis. Lancet. 2011;377(9775):1438–47. https://doi.org/10.1016/S0140-6736(11)60393-0
- Crimmins EM, Kim JK, Sole-Auro A. Gender differences in health: results from SHARE, ELSA and HRS. Eur J Public Health. 2011;21(1):81–91. https://doi.org/10.1093/eurpub/ckq022
- Dash SR, Hoare E, Varsamis P, Jennings GL, Kingwell BA. Sex-specific lifestyle and biomedical risk factors for chronic disease among early-middle, middle and older aged Australian adults. Int J Environ Res Public Health. 2019;16(2):224. https://doi.org/10.3390/ijerph16020224
- Adhikari K, Gupta N, Koshy AK. Gender differences on risk factors of noncommunicable diseases: a community based cross-sectional study in central Nepal. J Nepal Health Res Counc. 2014;12(27):89–93.
- Christiani Y, Byles JE, Tavener M, Dugdale PJ. Gender inequalities in noncommunicable disease risk factors among Indonesian urban populations. Asia Pac J Public Health. 2016;28(2):134–45. https://doi.org/10.1177/1010539515626265
- Eikemo TA, Gkiouleka A, Rapp C, Utvei SS, Huijts T, Stathopoulou T. Non-communicable diseases in Greece: inequality, gender and migration. Eur J Public Health. 2018;28(Suppl 5):38–47. https://doi.org/10.1093/eurpub/cky219
- McNamara CL, Toch-Marquardt M, Albani V, Eikemo TA, Bambra C. The contribution of employment and working conditions to occupational inequalities in non-communicable diseases in Europe. Eur J Public Health. 2021;31(1):181–5. https://doi.org/10.1093/eurpub/ckaa175
- Niedhammer I, Chastang JF, David S, Kelleher C. The contribution of occupational factors to social inequalities in health: findings from the national French SUMER survey. Soc Sci Med. 2008;67(11):1870–81. https://doi.org/10.1016/j.socscimed.2008.09.007
- Khorrami Z, Rezapour M, Etemad K, Yarahmadi S, Khodakarim S, Mahdavi Hezaveh A, et al. The patterns of non-communicable disease multimorbidity in Iran: a multilevel analysis. Sci Rep. 2020;10(1):3034. https://doi.org/10.1038/s41598-020-59668-y
- Alimohammadian M, Majidi A, Yaseri M, Ahmadi B, Islami F, Derakhshan M, et al. Multimorbidity as an important issue among women: results of a gender difference investigation in a large population-based cross-sectional study in West Asia. 2017;7(5):e013548. http://dx.doi.org/10.1136/bmjopen-2016-013548
- Handbook on health inequality monitoring with a special focus on low- and middle-income countries. Geneva: World Health Organization; 2013 (https://apps.who.int/iris/handle/10665/85345, accessed 8 December 2022).
- Noncommunicable disease surveillance, monitoring and reporting. STEPwise approach to NCD risk factor surveillance (STEPS). Geneva: World Health Organization (https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps, accessed 8 December 2022).
- WHO STEPS surveillance manual. The WHO STEPwise approach to noncommunicable disease risk factor surveillance. Geneva: World Health Organization; 2005 (https://apps.who.int/iris/handle/10665/43376, accessed 8 December 2022).
- Noncommunicable diseases and their risk factors: global physical activity questionnaire (GPAQ): analysis guide. Geneva: World Health Organization; 2021 (https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/physical-activity-surveillance, accessed 8 December 2022).
- Boehm JK, Soo J, Zevon ES, Chen Y, Kim ES, Kubzansky LD. Longitudinal associations between psychological well-being and the consumption of fruits and vegetables. Health Psychol. 2018;37(10):959–67. https://doi.org/10.1037/hea0000643
- Defining adult overweight and obesity [Internet]. Atlanta, GA: Centers for Disease Control and Prevention; 2022 (https://www.cdc.gov/obesity/adult/defining.html, accessed May 2021).
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–52. https://doi.org/10.1161/01.HYP.0000107251.49515.c2
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380(9859):2224–60. https://doi.org/10.1016/S0140-6736(12)61766-8
- Bregger JE, Haugen SE. BLS introduces new range of alternative unemployment measures. Monthly Lab Rev. 1995;118:19.
- Jann B. The Blinder-Oaxaca decomposition for linear regression models. Stata J. 2008;8(4):453–79. https://doi.org/10.1177/1536867X0800800401
- Rahimi E, Hashemi Nazari SS. A detailed explanation and graphical representation of the Blinder-Oaxaca decomposition method with its application in health inequalities. Emerg Themes Epidemiol. 2021;18(12):1–15. https://doi.org/10.1186/s12982-021-00100-9
- Rahimi, E, Hashemi-Nazari SS, Etemad K, Soori H. Decomposing gender disparity in total physical activity among Iranian adults. Epidemiol Health. 2017;39:e2017044. https://doi.org/10.4178/epih.e2017044
- Khuwaja AK, Kadir MM. Gender differences and clustering pattern of behavioural risk factors for chronic non-communicable diseases: community-based study from a developing country. Chronic Illn. 2010;6(3):163–70. https://doi.org/10.1177/1742395309352255
- Ahmadi A, Shirani M, Khaledifar A, Hashemzadeh M, Solati K, Kheiri S, et al. Non-communicable diseases in the southwest of Iran: profile and baseline data from the Shahrekord PERSIAN Cohort Study. BMC Public Health. 2021;21(1):2275. https://doi.org/10.1186/s12889-021-12326-y
- New data from WHO/Europe shows links between gender and noncommunicable diseases [Internet]. Copenhagen: World Health Organization, Regional Office for Europe; 2020 (https://www.who.int/europe/news/item/09-12-2020-new-data-from-who-europe-shows-links-between-gender-and-noncommunicable-diseases, accessed May 2022).
- George MS, Gaitonde R, Davey R, Sukumaran V, Mohanty I, Upton P, et al. Social networks and their impact on access to health care: insights from older widows living alone in Kottayam, South India. Ageing Soc. 2021:1–23. https://doi.org/10.1017/S0144686X21001100
- Kim KI, Lee JH, Kim CH. Impaired health-related quality of life in elderly women is associated with multimorbidity: results from the Korean National Health and Nutrition Examination Survey. Gend Med. 2012;9(5):309–18. https://doi.org/10.1016/j.genm.2012.08.001
- Alharbi BA, Masud N, Alajlan FA, Alkhanein NI, Alzahrani FT, Almajed ZM, et al. Association of elderly age and chronic illnesses: role of gender as a risk factor. J Family Med Prim Care. 2020;9(3):1684–90. https://doi.org/10.4103/jfmpc.jfmpc_1060_19
- Ainy E, Azizi FJ. Women, occupation and cardiovascular risk factors: findings from the Tehran Lipid and Glucose Study. Public Health. 2007;121(12):950–3. https://doi.org/10.1016/j.puhe.2006.12.016
- Ramazani Y, Karbasian N, Mobasheri MJ. A Survey on the state of physical activity among middle-aged women in health center in Zarin Shahr City in Iran, fall 2016. J Shahrekord Univ Med Sci. 2018;20(1):15–21 [In Farsi].
- Beyraghi N, Soklaridis S. Toward an understanding of the gender gap in Iran: Why health leaders should care and what they can do to close the gender gap? Iran J Psychiatry Behav Sci. 2019;13(1). https://doi.org/10.5812/ijpbs.64643
- Kim GR, Nam CM. Temporal trends in educational inequalities in non-communicable diseases in Korea, 2007–2015. PLoS One. 2017;12(12): e0190143. https://doi.org/10.1371/journal.pone.0190143
- Ahmed SM, Hadi A, Razzaque A, Ashraf A, Juvekar S, Ng N, et al. Clustering of chronic non-communicable disease risk factors among selected Asian populations: levels and determinants. Glob Health Action. 2009;2(1):1986. https://doi.org/10.3402/gha.v2i0.1986


